Supplementary Online Material Patients and Methods Patients and
advertisement

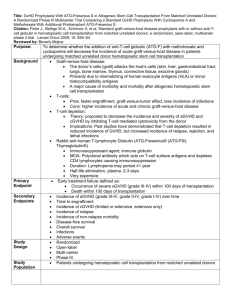
Supplementary Online Material Patients and Methods Patients and Transplantation Patients were identified from a database of 718 consecutive patients receiving allogeneic HCT in Calgary from January 1999 to March 2010. The rationale for starting in 1999 is that antithymocyte globulin (Thymoglobulin- Genzyme, Cambridge MA) was routinely used for GVHD prophylaxis at our institution since 1999 (Russell, J.A. et al.: Biol Blood Marrow Transplant 2007, 13:299-306). Also in approximately 1999, fludarabine and busulfan became the preferred conditioning regimen and filgrastim-mobilized blood stem cells became the preferred graft source. Moreover, our management of infectious complications of GVHD improved at approximately that time or thereafter. Specifically, preemptive therapy of cytomegaloviral disease started at our institution in 1999 and mold-active antifungals became available soon thereafter (caspofungin was Health Canada-approved in 2001 and voriconazole in 2004). Moreover new immunosuppressive therapies became available: mycophenolate mofetil was approved in 1998 and daclizumab in 2000. The reason for ending follow up in March 2010 was that we wished to determine the long-term survival, with minimum follow up of 2 years. Conditioning was typically with fludarabine (250 mg/m2), busulfan (approximately 12.8 mg/kg IV, pharmacokinetically adjusted) and ATG (4.5 mg/kg). Additional GVHD prophylaxis was with methotrexate (day 1, 3, 6, and 11) and cyclosporine from day -1 until 3 to 6 months posttransplant (longer in case of chronic GVHD) (Russel, J.A. et al.: Biol Blood Marrow Transplant 2007, 13:299-306). Conditioning of some patients included total body irradiation (4 cGy) (Russel, J.A. et al.: Biol Blood Marrow Transplant 2007, 13:814-821). Acute GVHD Treatment and Response to Treatment Acute GVHD (aGVHD) was graded by the Keystone Consensus criteria (Przepiorka, D. et al.: Bone Marrow Transplant 1995, 15:825-828). Patients were classified as steroid-refractory if they had no improvement or worsening of their overall aGVHD grade despite 2 mg/kg of prednisone or methylprednisolone (for a length of time determined by the attending physician), or if they had a recurrent flare of aGVHD while tapering or off corticosteroids that did not respond to incrementing or re-starting the corticosteroids. Patients were classified as steroidresponsive if they had complete and sustained resolution of aGVHD with only corticosteroid therapy. Steroid-refractory patients were typically treated with ATG (Thymoglobulin) at a dose of 2.5 mg/kg every other day for four doses, while the cyclosporine was held for 14 days and then reinstituted, and corticosteroids were continued. Response to ATG was evaluated at 4 weeks and 12 weeks and classified as complete (CR, achieving aGVHD grade 0), partial (PR, improvement in overall grade without deterioration in a single organ) or no response (NR, no change, worsening of overall grade, or improvement in overall grade but deterioration in a single organ). Literature Review of Survival after Grade 3-4 aGVHD Medline search was performed using subject heading “graft vs. host disease” and subheadings “mortality” and “therapy”. Studies cited in 2 recent reviews were also reviewed (Pidala J et al: Biol Blood Marrow Transplant 2010, 16:1504; Martin PJ et al: Biol Blood Marrow Transplant 2012, 18:982). Prospective as well as retrospective studies, regardless of therapy, were included if the outcome of patients with grade 3-4 (Consensus grading) or CIBMTR grade C-D aGVHD could be ascertained. These could be studies that either enrolled patients with any grade of aGVHD in which the outcomes of the patients with grade 3-4 aGVHD was presented or studies that included only patients with grade 3-4 aGVHD. Studies were included if they presented 10 or more patients and if surviving patients were either followed for at least 1 year (after transplant or after the initiation of therapy for aGVHD, whichever was given) or if Kaplan-Meyer survival estimate at >2 years was presented. Studies were excluded if they did not provide the length of follow-up time for surviving patients, unless Kaplan-Meyer survival estimate at >2 years was presented. If studies presented data for individual patients with variable follow-up times, those who were alive at the conclusion of the study but followed for less than one year were excluded while the remaining patients were included in the review. Supplementary Table 1. Patient Characteristics. Median Age, Years (Range) Steroid Responsive (N=12) 49 (27-61) Steroid Refractory (N=38) 46 (19-65) P-Value* 1.0 Sex (%) Female Male 4 (33) 8 (67) 14 (37) 24 (63) Diagnosis (%) Acute Leukemia MDS CML CLL Other 7 (58) 3 (25) 0 0 2 (17) 19 (50) 3 (8) 6 (16) 4 (10) 6 (16) HLA-Matched Sibling MUD HLA-Mismatched Unknown 3 (25) 6 (50) 3 (25) 0 (0) 15 (39) 9 (24) 13 (34) 1 (3) Stem Cell Source (%) Peripheral Blood Bone Marrow 11 (92) 1 (8) 33 (87) 5 (13) Conditioning Regimen (%) FLU+BU+ATG FLU+BU+ATG+ TBI Other** 4 (33) 8 (67) 21 (55) 15 (39) 0 (0) 2 (5) 22 (11-90) 33 (12-121) 3 11 (92) 21 (55) 4 1 (8) 17 (45) Donor (%) Median Posttransplant day of aGVHD onset (range) aGVHD Overall Grade (%) 0.45 0.27 0.41 1.0 0.23 0.11 0.03 0 (0) 3 (8) 1.0 Skin Only 0 (0) 1 (2) 1.0 Liver Only 3 (25) 17 (45) 0.32 GI Only 8 (67) 11 (29) Skin + GI 0.03 0 (0) 1 (2) 1.0 Skin + Liver 1 (8) 4 (11) 1.0 Liver + GI 0 (0) 1 (2) 1.0 Skin + GI + Liver *All P-values calculated via two-tailed Fisher’s exact test except for median post-transplant day of GVHD onset and median age - both calculated via Mann-Whitney U-test. ** Etoposide + total body irradiation + ATG in one patient, and Cyclophosphamide + total body irradiation + ATG in one patient. Abbreviations: MDS=myelodysplastic syndrome, CML=chronic myeloid leukemia, CLL=chronic lymphocytic leukemia, MUD=HLA-A,B,C,DRB1,DQB1-matched unrelated donor. aGVHD Organs Involved (%) Supplementary Table 2. Therapies given prior to or in conjunction with ATG in steroidrefractory aGVHD patients. Therapies Prior to ATG No. Corticosteroids Only 22 + MMF 9 + Tacrolimus 1 + Daclizumab 1 + MMF and Daclizumab 1 Therapies in Conjunction with ATG Corticosteroids Only 28 + Mesenchymal stromal cells (Prochymal, Osiris, Columbia, MD) or Placebo* 6 * Double-blind, randomized trial. Randomization status on individual patients is not available, however, it is irrelevant as the trial was negative (Martin, P.J. et al.: Biol Blood Marrow Transplant 2010, 16(2):S169-S170; Baron F & Storb R: Biol Blood Marrow Transplant 2012, 18(6):822-840). Supplementary Table 3. Overall response and response by organ to ATG at four and twelve weeks in patients with steroid-refractory graft-versus-host disease. Organ 4 Weeks 12 Weeks CR PR NR Death CR PR NR Death 11 (73) 1 (7) 1 (7) 2 (13) 7 (47) 0 (0) 0 (0) 8 (53) Skin (%) 6 (21) 9 (31) 7 (24) 7 (24) 8 (28) 1 (3) 4 (14) 16 (55) GI (%) 1 (17) 1 (17) 3 (50) 1 (17) 1 (17) 1 (17) 0 (0) 4 (66) Liver (%) 5 (15) 9 (26) 13 (38) 7 (21) 5 (15) 2 (6) 8 (23) 19 (56) Overall (%) Supplementary Table 4. Primary cause of death in steroid-responsive and in ATG-treated steroid-refractory aGVHD patients. Steroid-Responsive ATG-Treated SteroidRefractory aGVHD 0 3 aGVHD + Infection 0 6 Infection 3 11* Chronic GVHD 4 3 Relapse 3 3 Other 0 3** Unknown 0 1 * Including 2 cases of post-transplant lymphoproliferative disorder. ** Cerebral bleeding in one case, myocardial infarction in one case, and thrombotic microangiopathy in one case Supplementary Figure 1. Survival plot for patients with steroid-refractory and steroidresponsive grade 3-4 acute graft-versus-host disease. 100 80 60 Steroid-Responsive Steroid-Refractory 40 20 0 0 1000 2000 3000 4000 5000 Days from aGVHD onset