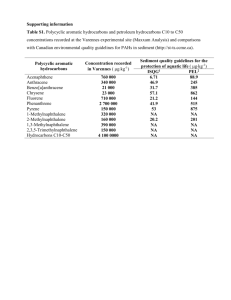
SME_SMEcl Scientific Principles and
advertisement

SME & SMEcl Technologies Scientific Principles and Application The following material was developed by Mark Ellis, Ellis Environmental, Lehi, Utah Subsurface metabolic enhancement (SME) is a biological method of remediating petroleum based hydrocarbons and chlorinated solvents in soils and groundwater. The method is practiced in-situ and focuses on changing the subsurface respiratory environment from anaerobic to aerobic. Two forms of SME are in current use; SME for petroleum based hydrocarbons and SMEcl for chlorinated solvents. The key factor distinguishing SMEcl from other types of biological remediation is that the bacteria used, Ralstonia eutropha var. AEK301/pYK3021, does not cometabolize chlorinated solvents in steps. Therefore it does not create daughter products, which are more toxic than the original contaminates. SMEcl will be described concurrently with the basic theory and practice of SME. The SME technologies were patented by Mark T. Ellis of Ellis Environmental located in Lehi, Utah. Pure Environmental Management, LLC (PEM) and Ami Adini & Associates, Inc., have agreements to market and use the technologies. Many of the cited projects and development refer to work done by Ellis Environmental. The method utilizes low pressure/low volume air exchange to remove contaminants from the subsurface soil and groundwater biologically. SME uses four components to effectively remediate hydrocarbons: 1. molecular oxygen transfer to the groundwater and soil; 2. subsurface injection of nutrients to feed microbes; 3. waste gas extraction through a process referred to as Biogenic Gas Extraction (BGE); and 4. a surface seal over the treatment area. Oxygen transfer and delivery of nutrients is accomplished through a grid work of vertical piping advanced into the affected zone. Spacing of the delivery grid is dependent on soil characteristics. Extraction of CO2 is accomplished through a separate grid work of 2-inch slotted and blank PVC piping, which may be vertical or horizontal depending on site conditions. Definitions ADP - adenosine diphosphate, substrate on which ATP is built for energy extraction in the mitochondrium AMU - atomic mass unit Anoxic - without oxygen Aquitard - a relatively impermeable confining layer of fine-grained soil or bedrock that impedes downward migration of water or other liquid ATP - adenosine triphosphate, the molecule that delivers energy to cells, made in the mitochondrium Aerobic - respiratory process that requires oxygen Anaerobic - respiratory process that operates in the absence of oxygen BGE - biogenic gas extraction, removal of CO2 from the subsurface SME Biofilm - a massive colony of single-celled microbes that form to consume an abundance of nutrients Bioremediation - using biological methods to degrade, remove or treat contamination Biosparge - bubbling air through water for the purpose of aeration CAP - corrective action plan CGI - combustible gas indicator DRO – diesel-range organics Glycolysis, glycolytic - breaking down glucose for metabolism Enzyme - complex protein that facilitates biochemical reactions without requiring additional heat input GRO – gasoline-range organics Header - a structure that collects air from smaller extraction structures, e.g., BGE system; the header collects air from wells and conveys the air to the blower for emission to the atmosphere HVAC - heating, ventilating and air conditioning IEPA - Illinois Environmental Protection Agency Injector - a mechanical device that permits the dispersion of gas or liquid in the saturated or unsaturated subsurface environment In-situ - in place LEL - lower explosive limit MCL - maximum contaminant level for water contaminants, established by EPA & State agencies Metabolism - extracting energy from food inside the body Microbe, microbiota, microorganism - single celled organisms such as fungi, bacteria or algae that are generally too small to see with the human eye without magnification Mitochondrium, mitochondria - singular and plural forms, describes an organelle that produces energy for living cells Molecular Oxygen Transfer - a phase shift of injecting oxygen as air and a gas into groundwater, dissolving the oxygen so that it becomes part of the water matrix MtBE - methyl tertiary butyl ether, a gasoline additive NFA - no further action, issued by the regulatory agency to indicate completion of remedial action O&G - oil & grease Oxidative Phosphorylation - synthesis of ATP from ADP for which energy is obtained by electron transfer, an aerobic respiratory process Phenol hydroxylase - enzyme that allows Ralstonia eutropha to debond chlorine atoms from a carbon-based substrate of chlorinated hydrocarbons Ralstonia eutropha - R. eutropha, var. AEK301/pYK3021 a pseudomonad bacterium modified to possess the enzyme phenol hydroxoylase Respiration - the metabolic process of extracting energy from food, resulting in the excretion of carbon dioxide (aerobic metabolism) PCE - perchloroethylene, perc, tetrachloroethylene or tetrachloroethene SME - Subsurface Metabolism Enhancement, U.S patent #6,464,005 SMEcl - SME applied to degrading chlorinated hydrocarbons Stoichiometric - quantitative relationship between chemicals in a given chemical reaction SVE - soil vapor extraction TCE - trichloroethylene or trichloroethene TEFC - totally enclosed, fan cooled (electrical motor) TPH - total petroleum hydrocarbons TSDF - treatment, storage or disposal facility of hazardous waste UST - underground storage tank as regulated by 40 CFR 280 VOC - volatile organic compounds Bioremediation Principles and Evidence Bioremediation is chosen over chemical remediation for a number of reasons. Bioremediation eliminates the source of contamination by changing contaminating molecules to non-toxic forms using processes of biological metabolism. In this manner, the contamination is not transferred to another medium, moved around in the subsurface or hidden; it is consumed. Bioremediation operates in the groundwater as well as the soil where SME is installed. SME bioremediation is a completely biological method of remediation, not a biological byproduct of a mechanical or chemical method; it is a natural degradation system employing natural means. SMEcl varies from the SME methodology only in that it injects R. eutropha and a supplementary food base. Following is a description of bioremediation principles that apply to SME and SMEcl. Researchers in the nineteenth and early twentieth centuries were fully aware that microorganisms consume petroleum. In 1946, Claude E. Zobell1 produced a paper that explored the degradation of petroleum by microorganisms. He cited numerous investigations conducted decades before his time describing various microorganisms that used petroleum products or crude oil as either an energy source or a growth substrate. Below are examples of just a few of the investigations. Most of these investigations were written in German, so they are reproduced here as cited by Zobell: Miyoshi reported2 in 1895 that thin layers of paraffin were penetrated by Botrytis cinerea. Rahn reported3 in 1906 that fungi as various soil molds attacked paraffin, including Penicillium glaucum, using paraffin as its sole source of energy. Söhngen showed4 that paraffin was attacked by 17 different species of soil bacteria, which also used petroleum ether, paraffin oil, crude petroleum and caoutchouc. Störmer reported5 in 1908 that Bacillus hexacarbovorum, metabolizes toluene and xylenes. Wagner described6 Bacterium benzoli a and b, which utilized benzene, toluene, xylenes and various aliphatic hydrocarbons for energy; he wrote in 1914. Tausz and Peter studied7 Bacterium aliphaticum liquefaciens, that attacked a wide range of hydrocarbons, including caprylene, hexadecene, cyclohexane, methylcyclohexane and paraffinic hydrocarbons ranging in size from n-hexane to tetratriacontaine (C34H70). They reported their findings in 1919. In a more recent analysis, Atlas8 cited at least 18 investigators/investigations where large lists of fungi, bacteria and even some algal genera were found to metabolize petroleum hydrocarbons. Hydrocarbons have always been a part of our natural environment. All life forms contain hydrocarbons, and in general, material that contains hydrocarbons is called organic material.9 ZoBell (1946) observed that 0.02% of all solids produced by plants are hydrocarbons. Some plants have been found to be rich in oil. For example, Meadowfoam plants10 have a measured mean oil concentration of 29% while the seeds have a measured mean oil concentration 31.6%. Mean oil concentration11 of Dimorphotheca pluvialis has been measured at 21.5%. It follows that microorganisms are available to degrade these hydrocarbons just like more concentrated forms of crude-oil hydrocarbons can be degraded or piles of indigestible plant products would eventually cover the earth. Martin et al.12 showed a large list of non-methane hydrocarbons, monocarboxylic acids and low molecular weight carbonyl compounds that are emitted by deciduous and coniferous vegetation. The natural and continuing sources of hydrocarbons have a complementary niche of microorganisms that metabolize hydrocarbons as an energy source. A variety of lithogenic hydrocarbon sources have exposed the earth’s surface to organic compounds through oil seeps, oil shales, coal deposits, etc. Natural seeps of hydrocarbons documented in the Prince William Sound, Alaska have been known13 at that location for at least 160 years according to soil core analyses. The seeps do not cause an accumulation of hydrocarbons. The hydrocarbons are being degraded by the natural ecosystem. Natural oil shale that is so abundant in the western United States was seriously considered as a petroleum source during the energy crunch of the 1970's and is once again being evaluated for producing domestic fuel. As a result of widespread contamination created by a federal government retort 14 research facility, the State of Wyoming 15 required the Department of Energy to remediate hydrocarbons in the Tipton aquifer near Rock Springs, WY. The hydrocarbons originated from the oil shale treated at their oil shale retort experiment facility. A host of microorganisms exist that utilize lithogenic hydrocarbons for energy, as described in the proposal for sparging the Tipton aquifer. The 2010 spill from a British Petroleum America, Inc. platform, Deepwater Horizon in the Gulf of Mexico was reported to have “lost” a significant portion of the crude oil spill, reported 16 at 4.9 million barrels. When oil skimmers were put on the spill, they reported finding less and less oil to skim, not due to the skimming. John Carey reported 17 that after a month or two of the spill, microbes will have consumed up to half of the spilled oil. Natural bioremediation takes advantage of the native microbiota that consume hydrocarbons as a source of energy, regardless of the origin. To make optimum use of the microbial communities, SME improves the subsurface environment for aerobic microbes and essentially “farms” the microbes. By improving conditions for the microbes, hydrocarbons are consumed and converted naturally to harmless carbon dioxide and water. SMEcl Operating Principles Chlorinated hydrocarbons are typically heavier than water. The specific density of the chlorinated hydrocarbons is a function of the chlorine atom, which at 35 amu, possesses 35 times the mass of hydrogen and 3 times the mass of carbon. Attaching chlorine to carbon simply makes the molecule heavy. Most hydrocarbons have a specific density less than 1 (the density of water), e.g., benzene has a specific density of 0.8787 at 15°C; hexane has a specific density of 0.6591 at 20°C; gasoline has an approximate density ranging from 0.7 - 0.8 and diesel fuel has an approximate density ranging from 0.8 - 0.88 at 15.6°C. These lighter-than-water specific densities mean that the contamination is going to be found near the top of the groundwater aquifer. Treatment for hydrocarbons focuses on the top of the aquifer, the capillary fringe and the smear zone soil that absorbs the contamination as the aquifer changes elevation. Most chlorinated hydrocarbons have a greater-than-water specific density. TCE has a specific density of 1.4695 at 15°C. PCE has a specific density of 1.6311 at 15°C. The cis- and trans- forms of 1,2-dichlorothene have a specific gravity of about 1.28. A notable exception is vinyl chloride, a particularly poisonous chlorinated ethene with a specific gravity of 0.9106 at 20°C. Specific gravity provides that compounds with heavier-than-water gravities will not generally remain at the surface of the aquifer. The dense, chlorinated hydrocarbons tend to migrate to the aquitard, at the bottom of the aquifer. They will leave a trail through the soil and surface groundwater as they migrate, but monitoring or testing for these dense liquids in the same, top of aquifer location and in the same manner, as hydrocarbons will not provide accurate information. If anaerobic processes are used to degrade the chlorinated hydrocarbons, the chlorinated hydrocarbons are degraded in steps as illustrated below. As the anaerobic process unwinds, the compounds become lighter (and more poisonous) until the single-chlorine compound vinyl chloride is produced. This compound is lighter than water and might float into other areas of the aquifer, not staying with the heavier compounds that migrate to the aquitard. If the progression of the anaerobic bioremediation process is interrupted or stopped, it is conceivable that the highly poisonous vinyl chloride could be released from the aquitard and make the original chlorinated hydrocarbon release much more complicated, not to mention more dangerous. PCE→ TCE→ cis or trans 1,2 →DCE → Vinyl Chloride → Ethene The principles of SME begin with molecular oxygen transfer. In the native environment that becomes contaminated with chlorinated hydrocarbon or hydrocarbons, the native microbes are flooded with an excess of food. As the microbes commence metabolism of the organic contamination, oxygen is quickly used up and the microbes stop consuming the hydrocarbons aerobically. Microbes can consume the contamination anaerobically, but at a much slower rate. Air injection infuses the subsurface with oxygen, converting the glycolytic pathway of cellular respiration to the more efficient aerobic respiration. The process with chlorinated hydrocarbons is more complicated than with hydrocarbons. R.eutropha is able to use the phenol hydroxylase to insert an oxygen molecule into the double carbon bond, which breaks down the molecule and allows the chlorine to de-bond from the carbon molecule. There are no other steps and no other enzymes needed in the aerobic process. Note that the aerobic process that R. eutropha uses is far less complicated and more rewarding. There are no intermediary steps as is required with the anaerobic process. One must wonder what the incentive is for anaerobic bacteria to degrade the chlorinated hydrocarbons if only the last process yields any carbon for consumption. The aerobic process is far more efficient. Under aerobic conditions, hundreds of bacteria, fungi and algae are capable of metabolizing a huge range of hydrocarbons. Many of the naturally occurring microbes that consume hydrocarbons are facultative aerobes, allowing them to change their respiration from anaerobic to aerobic. Generally speaking, when cellular respiration converts from anaerobic to aerobic, energy production changes from two units of adenosine triphosphate (ATP) per acetate molecule to as much as 36 units of ATP per molecule. Not all of the ATP is used by the cell for energy, but simply put, aerobic respiration is at least a dozen times more energy efficient than anaerobic glycolysis. There are reports of anaerobic and aerobic processes that degrade chlorinated hydrocarbons. However, with the singular exception of the phenol hydroxylase method, all other methods require a co-metabolite or a step-wise progression to achieve full contaminant metabolism. R. eutropha requires no metabolite to activate the enzyme phenol hydroxylase. Aerobic respiration generally involves metabolizing the hydrocarbon molecule for the energy in the carbon. After the microbes metabolize the carbon, they excrete carbon dioxide and water. Oxidative phosphorylation is the aerobic, cellular process that extracts energy from carbon molecules using oxygen. The microbes ingest the carbon, transform the carbon to glucose, then extract energy in the form of Adenosine Triphosphate (ATP). It is a complex process requiring enzymes and specific organic complexes. The process may differ somewhat from higher to lower organisms, but the essential process is the same; ingested carbon is converted through cellular respiration (metabolism) to energy, allowing biomass to increase and more reproduction to occur. In this case, the energy that drives metabolism is provided by hydrocarbons. The chemical balance of hexane breakdown is shown here as an example of the fate of hydrocarbons when completely metabolized. To metabolize one mole of hexane requires 9.5 moles of oxygen. C6 H14 + 9.5 O2 → 6 CO2 + 7 H2O Research has found that one-fourth of the available carbon is used to produce microbial mass18 (reproduction), the remaining carbon is excreted as carbon dioxide (CO2). Respiration from the microbes generates biogenic CO2 that accumulates in the subsurface environment. Respiration takes place in the soil, just as in the groundwater. Using this information, we are able to develop calculations to determine how much hydrocarbon is actually metabolized in a remediating site. Typically, the measurements for the calculations are made in the BGE air stream. Each biosparge point injects nine pounds of oxygen into the subsurface daily. This assumes a flow rate of 20 cfh or 566 L/hr, oxygen concentration in air of 21%, molecular weight of oxygen molecule (32), gas law of 32 g O2/ 22.4 L air and conversion factors as shown. 566 L / hr x 24 hr / day x 0.21 L O2 x 1 L air 32g O2 x 1 lbs = 9 lbs O2 / day 22.4 L air 454 g The SMEcl biosparge system is advanced into the zone where the chlorinated hydrocarbon contaminants are located, at the aquitard. The biosparge system is controlled to emit only sufficient air to dissolve the oxygen into the groundwater. There is no attempt to strip out contamination by aggressive sparging. The result is a biological ecosystem that becomes aerobic and able to thrive with a constant flow of dissolved oxygen in the previously anoxic environment. The disturbance to the public resulting from the installation and operation of air injection is minimal. The installation time for a single SME circuit is one week from start to finish. Tubing and piping are laid in narrow trenches ranging from 1.5 to 14 inches wide. Typically, businesses remain at nearly full operation during the entire installation procedure. The public is excluded from the installation area, but there is no limitation of property use and access once the air injectors are in place. The BGE System Without the removal of the waste gas CO2, bioremediation does not completely remove all contamination. The accumulation of CO2 in the subsurface poisons the microbes, stopping them from metabolizing hydrocarbons, either aerobically or anaerobically. For this reason, air injection as used in the SME method is paired with a gas extraction system. Because the CO2 is biogenically derived, the extraction system is referred to as the biogenic gas extraction (BGE) system. BGE uses a low-pressure regenerative blower, operated in vacuum mode and rated for high volume relative to the negative pressure exerted in the subsurface. The objective of soil gas extraction as used by BGE is to remove metabolic waste products such as CO2 out of the soil. CO2 generated in the aquifer comes readily out of solution and emerges into the soil column. At that point, CO2 can be collected by the BGE and emitted into the atmosphere. The BGE does not target removal of hydrocarbon vapors, although start up of BGE will invariably pick up some light-end hydrocarbons. Emission of light-end hydrocarbons from the BGE typically lasts 2-3 weeks after which the hydrocarbon emissions virtually cease. BGE differs from conventional SVE by removing soil gas, particularly CO2 by airflow, not by high-pressure differential. Air movement in soil is influenced by diffusion, mass flow, moisture content and atmospheric pressure19. Mass flow (first of Fick’s two laws20 of gas movement and mass flow) is caused by pressure differences between the soil and atmosphere and is enhanced by moisture content, i.e., increase the moisture in the soil and the pressure in the soil increases, which forces air out of the soil. The second of Fick’s laws describes removal of one molecule in the soil matrix, which is then replaced by another molecule. Therefore, if low-pressure air extraction removes a volume of air, it is replaced by a similar volume of air from elsewhere. When pressure is applied to fine grained soil, the force of the air extraction results in soil resorting and channeling, restricting the primary goal which is soil-air flow. When pressure is reduced and the focus becomes air flow, the structure of the soil becomes less of an issue. With pressure reduction, soil does not resort, does not collapse and allows the gas exchange to take place that biological systems depend upon. If sand has a pore space of 15% and clay has a pore space of 45%, why should air not move through clay, but only through sand? The convoluted fragments of soil particles in fine soils like clay still transmit air at the molecular level because Fick’s laws work. Fick’s laws make two things very clear; first, that high pressure in the subsurface is unnecessary in order to move air through the soil and second, air flow is not restricted to only coarse grained soil. The most effective and least destructive means of removing CO2 from the subsurface is to decrease air flow and negative pressure so that diffusion and mass flow can occur. BGE can selectively remove carbon dioxide over hydrocarbon by taking advantage of the physical properties of those compounds. Carbon dioxide has a much higher vapor pressure than does petroleum, even than that of gasoline. With a vapor pressure of approximately 856 PSI (70°F), CO2 exerts a much greater pressure than does gasoline, which has a vapor pressure range of 9-15 PSI. In the subsurface, this means that CO2 is about 100 times more “active” in the soil and will preferentially migrate as mass flow towards the BGE extraction system. This is borne out in SME measurements, which show that even with a much larger extracted-volume-of-air-to-injected-air, the majority of the petroleum in the contaminant plume is removed by the process of metabolism, not vapor extraction. Illustrative of the hydrocarbon metabolism vs. emitted VOC ratio, Ellis Environmental has generated data at current and past SME treatment sites. The data show that on average, 79% of hydrocarbons are metabolized in the subsurface; the remainder is emitted as VOC at the start up of the project. BGE used with chlorinated hydrocarbons does not result in emissions of chlorine. Chlorine is a very reactive element, which is part of the reason why chlorine is considered so dangerous. That reactivity means that the free chlorine liberated from the cleaving action of R. eutropha will react in the subsurface. The chlorine binds with basic cations in the soil to create a salt. Nutrient injection. Nutrients essential for the formation of body mass and metabolic processes of microbes are very simple, since bacteria manufacture many of their own nutrients (vitamins) using basic raw materials. Carbon and oxygen are not nutrients; carbon is used as the energy source and oxygen is the oxidizer as described above. Nutrients for single cell organisms need a nitrogen source such as ammonia, nitrate and urea. Phosphorus is needed, usually in the form of phosphoric acid or the ortho configuration of phosphate. As mentioned in the previous paragraph, carbon is not a nutrient in the classical sense. It is an energy base. Bacteria that are injected to the subsurface to remove contamination cannot be assumed to be living solely on the target contaminants. A dilute contaminant will not support growth and survival of the bacteria unless there is sufficient carbon available for a growing colony. Unlike the injections of ignitable methane and hydrogen used for anaerobic bacteria, R. eutropha will feed on non-ignitable carbon sources. Carbon injections for R. eutropha are not cometabolites, they are food sources. The bacteria will survive regardless of whether additional carbon is injected or not. However, if the goal is to encourage the rapid metabolism of chlorinated hydrocarbons, additional carbon or food is used to grow the R. eutropha colony, which will consume the contaminants as those are encountered. When microbes metabolize hydrocarbons, nutrients and especially nitrate21 are rapidly depleted. As early as 1928, Tausson, a Russian researcher, determined22 that a source of nitrogen such as ammonium or nitrate ions was one of four necessary ingredients for microbial degradation of oils. When the supply of nitrogen is limited, metabolism slows or halts altogether, even in the midst of a rich energy source such as a hydrocarbon plume. The necessity of nutrients in bioremediation was highlighted in the research23 by Albert Venosa, et al. at Delaware Bay. Venosa’s work was intended to demonstrate the effect of bacteria and/or nutrients on degradation of hydrocarbons in open water. His methods and conclusions showed that native microflora use nitrate to consume hydrocarbons. Mihelcic and Luthy reported24 that 0.8 moles nitrate were required for each mole of carbon dioxide produced when petroleum is metabolized. Nitrate and a few forms of lithogenic nitrogen are found only in low concentrations25 in natural water and usually less than one mg/L in natural groundwater, which is not affected26 by human activities. Ideal ratios for carbon:nitrate:phosphate concentrations are documented for aquatic microflora. Nutrient ratios for aquatic macrophytes and algae are reported27 to be 40:7:1. Bossert and Bartha found28 that the ideal carbon:nitrogen:phosphorus ratio in soil is 160:1:0.08. Phosphates may not be abundant in midwestern U.S. soil and could also be injected into the subsurface. As a side note, injected nutrients do not spread well, so relying on nutrient injection, as a sole remediation method requires an intensive distribution system. Positive results from nutrient injection are limited to portions of the plume exposed to the nutrient. Nitrates injected to the subsurface in high concentrations tend to develop biofilms of bacterial colonies in the soil that trap the nutrients and soil gases, preventing them from spreading. The biofilm tends to reduce groundwater movement and reduce the effectiveness of any remedial action. Biofilm development is usually the result of too high a concentration of nitrates injected without a dispersal mechanism. The nutrients most lacking in an exploding population of microbes are nitrogen, phosphorus and potassium. SME adds nutrients that are required to build and maintain a healthy population of microbes capable of metabolizing hydrocarbons. The nutrient amendments usually include nitrogen in the form of ammonium nitrate, ammonium sulfate, urea or ammonia. Phosphates are added as phosphoric acid. Micronutrients may be added, depending upon the results of the inorganic analyses provided with each site investigation. Surface Seal SME works much more efficiently and economically when a relatively impermeable barrier seals the ground surface above the plume. The surface seal is not as critical when the target plume is located at the bottom, rather than the top of the aquifer. When the target is hydrocarbons, a seal of asphalt or concrete are typically used at commercial or industrial properties. All subsurface air extracted by the BGE is provided either by the air injectors or air leakage from the surface. The surface seal forces the air moving from the surface into the BGE to travel a long distance. The balance of air volume extracted to injected is typically in the range of 5: 1to 10:1. PEM gauges the effectiveness of SME by measuring O2, CO2 and VOC in the BGE emission stack(s), using a combustible gas meter. What we look for in the O2 measurement is the depression of O2 below background, calculated at 21%. Oxygen depression is a measurement of actual oxygen consumed in the metabolic process and is a more reliable measure of consumed hydrocarbons than is a direct measurement of CO2. This is because metabolic activities in alkaline soils will often include a break down of carbonates and bicarbonates in the soil to CO2, creating false positive CO2 values and generating a larger emission of CO2 than what is generated by the metabolism of hydrocarbons. CO2 measurement in the SME system is used to assure that metabolism is continuing. When the concentration of CO2 in the BGE emissions goes to or near zero, which is an indication that the energy source as hydrocarbons may be exhausted. VOC is measured as a general indicator of emissions, but this figure is rarely used to evaluate bioremediation progress. Airflow measurement cannot be performed in the same manner with BGE as with SVE. Conventional SVE systems develop high vacuum (negative pressure) and are not expected to be dispersed over a large volume of the subsurface. SVE systems tend to form channels in the subsurface soil. SVE systems typically draw 100% of replacement air from the surface. The subsurface air is extracted under high volume and with high negative pressure. Surface air leaks into the subsurface, following the path of least resistance. High flow rates create channels, where soil is either compressed to form the channels or the soil is vacuumed out of the ground to make the channels. Air movement through the soil is mostly vertical, giving a very small radius of influence. Many SVE systems are designed with filtration devices to minimize damage to air extraction equipment. On the other hand BGE collects the low volume air input from air injection (10 to 20%) and the balance of the extracted air coming from surface leaks. By keeping the pressure low enough to prevent channeling, the radius of influence is considerably larger, particularly when a good surface seal is present. The BGE system influence in the subsurface is not measured by negative pressure, but by airflow. A qualitative measure of airflow is a smoke test. This method is used in the HVAC industry and is valid in this application. An air test well may be constructed near the BGE system, no more than 40 feet away and closer if possible. Well screen needs to be located in the vadose zone at the same elevation or deeper than the well screen of the gas collector. With the collector system fully activated, employ a smoke-generating device, held over the open end of an air test well. If the smoke is drawn into the air test well, airflow is thereby shown to move from the surface, into the ground, through the soil and into the collector system. If the smoke is not drawn into the well, there is no movement in the subsurface air at the location of that test well. A major consideration that advocates SME for remediating hazardous material spills is that this biological treatment generates no hazardous waste. The hazardous material is treated in place, it only generates an emission of carbon dioxide and requires little in terms of surface disturbance for installation of the treatment system. SMEcl shifts the hazardous waste disposal paradigm because there are/is no: waste characterization samples to be collected and analyzed, - waste disposal applications to submit, insurance required for long term liability, - manifest to transfer, lose, replace or cause difficulty with the regulating agency, - cradle to grave liability for the generated hazardous waste, nor is there a life long liability to the generator of the waste, concern regarding the viability of the TSDF, wondering if future liability is going to appear based upon past-complying actions, - toxic or noxious residue left in the subsurface following treatment. SMEcl is the ultimate hazardous waste minimization strategy, taking pressure off decreasing capacities at landbased TSDF. SMEcl reduces liability errors that may occur at treatment facilities such as incinerators or chemical stabilizers. Most hazardous waste industry professionals understand that eliminating hazardous waste liability is an enormous business advantage. 1 Zobell, Claude E., Action of Microorganisms on Hydrocarbons, Bacteriol. Rev. 10:1-49, 1946 2 Miyoshi, M. 1895 Die Durchbohrung von Membranen durch Pilzfaden. Jahrb.wiss. Botan., 28, 269-289. Cited by ZoBell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 3 Rahn, O. 1906 Ein Paraffin zersetzender Schimmelpilz. Zentr. Bakt. Parasitenk. Infek., II, 16, 382-384. Cited by ZoBell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 4 Söhngen, N.L., 1913a. Benzin, Petroleum, Paraffinol und Paraffin als Kohlenstoff-und Energiequelle fur Mikroben. Zentr. Bakt. Parasitenk. Infek., II, 37, 595-609. Cited by ZoBell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 5 Störmer, K. 1908 Ueber die Wirkung des Schwefelkohlenstoffs und ähnlicher Stoffe auf den Boden. Zentr. Bakt. Parasitenk. Infekt., II, 20, 282-286. Cited by Zobel, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 6 Wagner, R. 1914. Über Benzol-Bakterien. Z. Gärungsphysiol. 4, 289-319. Cited by ZoBell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 7 Tausz, J. und Peter, M. 1919 Neue Methode der Kohlenwasserstoffanalyse mit Hilfe von Bakterien. Zentr. Bakt. Prasitenk. Infek., II 49, 497-554. Cited by ZoBell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 10:1-49, 1946. 8 Atlas Ronald M., Microbial Degradation of Petroleum Hydrocarbons: an Environmental Perspective, Microbiological Reviews, Vol. 58, No. 1, March 1981, pp. 180-209. 9 http://encarta.msn.com/encyclopedia_761558734/Coal.html 10 Patrick, B.E. & G.D. Joliff. Oil Content Distribution of Meadowfoam Seeds by Near-infrared Transmission Spectroscopy. p. 234-236. In: J. Janick (ed.), Perspectives on new crops and new uses. ASHS Press, Arlington, VA, 1996. 11Hof, L. Dimorphotheca pluvialis: A new source of hydroxy fatty acid. p. 372-377. In: J. Janick (ed.), Progress in new crops. ASHS Press, Arlington, VA, 1996. 12 Martin, Randal S., Ignacio Villanueva, Jingying Zhang and Carl J. Popp, Nonmethane Hydrocarbons, Monocarboxylic Acid, and Low Molecular Weight Aldehyde and Ketone Emissions from Vegetation in Central New Mexico, Environmental Science and Technology, Vol. 33, No. 13, 1999, pp. 2186-2192. 13 Boehm, Paul D., et al., Resolving the Origin of the Petrogenic Hydrocarbon Background in Prince William Sound, Alaska, Environmental Science and Technology, Vol. 35, No. 3, 2001, pp 471-479. 14 Read about retorting oil shale at http://ostseis.anl.gov/guide/oilshale/ 15 Environmental Assessment, Remediation of Subsurface And Groundwater, Contamination at the Rock Springs in Situ Oil Shale Retort Site, U.S. Department of Energy, National Energy Technology Laboratory Sweetwater County, Wyoming. http://nepa.eh.doe.gov/ea/ea1331/ea1331.pdf. 16 http://en.wikipedia.org/wiki/Deepwater_Horizon_oil_spill 17 http://news.yahoo.com/s/ynews_excl/ynews_excl_sc3270 18 Hinchee, Robert E. and Say Kee Ong, "A Rapid In Situ Respiration Test for Measuring Aerobic Biodegradation Rates of Hydrocarbons in Soil", Journal of Air and Waste Management 19 Brady, Nyle C., The Nature and Properties of Soils, Macmillan Publishing Company, New York, 1990, pg. 154. 20 Fick’s laws may be reviewed at https://secure.wikimedia.org/wikipedia/en/wiki/Fick's_law_of_diffusion 21Carman, Kevin R., T.S. Bianchi, F Kloep, Influence of Grazing and Nitrogen on Benthic Algal Blooms in Diesel FuelContaminated Saltmarsh Sediments, Environmental Science & Technology, Vol. 34, No.1, pp 107-111, 2000. 22 Cited by ZoBell, Claude E., Action of Microorganisms on Hydrocarbons, Bacteriol. Rev. 10:1-49, 1946. Refer to Tausson, W.O., 1928a, Bacterial Oxidation of Crude Oils, Neftyanoe Khoz., 14, 220-230 (in Russian). 23 Venosa, Albert D. et al., Bioremediation of an Experimental Oil Spill on the Shoreline of Delaware Bay, Environmental Science and Technology, Cincinnati, OH; Vol 30, No. 5, pp. 1764- 1775, 1996. 24 Mihelcic, James R. and Richard G. Luthy, Microbial Degradation of Acenaphthene and Naphthalene under Denitrification Conditions in Soil-Water Systems, Applied and Environmental Microbiology, May 1988, pp. 1188-1198. 25 Hem, John D., Study and Interpretation of the Chemical Characteristics of Natural Water, Geological Survey Water-Supply Paper 1473, United States Government Printing Office, Washington: 1970. 26 11/3/92 personal conversations between John Turk and Stan Robeson of USGS, Denver office, tel. 303-236-4882 and Mark Ellis of Ellis Environmental 27 Wetzel, Robert G., Limnology, page 640, W.B. Saunders Company, West Washington Square, Philadelphia, PA 19105. 28 Petroleum Microbiology, R.M. Atlas, Editor. MacMillan Publishing Co., NY. Quoted from Bossert, I. and Bartha, R., 1984. The Fate of Petroleum in the Soil Ecosystem. In: Cleanup of Releases from Petroleum USTs: Selected Technologies, USEPA, April 1988.