Natures Chemistry Hydrocarbons N5 Homework One The names of
advertisement

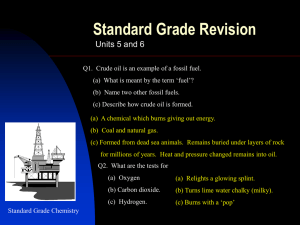
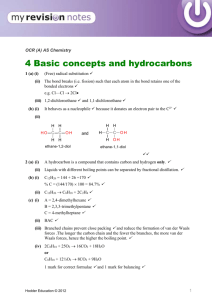
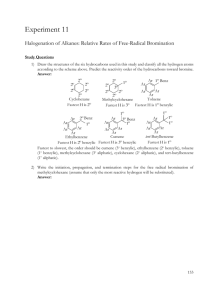
Natures Chemistry Hydrocarbons N5 Homework One 1. The names of some hydrocarbons are shown in the grid. (a) Identify the hydrocarbon with the highest boiling point. (You may wish to use the data booklet to help you). (b) Identify the two isomers. (c) Identify the two hydrocarbons which can take part in addition reactions with hydrogen. 2. The names of some hydrocarbons are shown in the grid. (a) Identify the hydrocarbon which is a liquid at 25˚C. (You may wish to use the data booklet to help you). (b) Identify the two isomers. (c) Identify the hydrocarbon that reacts quickly with bromine solution. 3. The grid shows the formulae of some hydrocarbons. (a) Identify the two cycloalkanes. (b) Identify the structural formula which represents the first member of a homologous series. 4. Hydrocarbons contain hydrogen and carbon only. (a) Identify the two hydrocarbons which would quickly decolourise bromine solution. (b) Identify the isomer of the hydrocarbon in box D which belongs to a different homologous series. 5. Hydrocarbons compounds made from hydrogen and carbon only. (a) Identify the hydrocarbon which reacts with hydrogen to form butane. (b) Identify the two hydrocarbons which are the first members of a homologous series. (c) Identify the two hydrocarbons with the general formula Cn H2n which do not react quickly with bromine solution. 6. Methane (CH4), ethane (C2H6), and propane (C3H8) are the first three members of the alkanes. (a) State the general formula for the alkanes. (b) The ninth member of the alkane is nonane (C9H20). The table gives some information about other members of the alkane family. Predict the boiling point of dodecane. (c) The alkanes are said to be members of a homologous series. What is meant by a homologous series? 7. Propene has the structural formula shown below. (a) Draw the shorten structural formula for propene. (b) Propene quickly decolourises bromine water, (Br2(aq)). (i) Name the type of reaction which takes place when propene reacts with bromine water. (ii) Draw the full structural formula of the product of the reaction. 8. The octane number indicates how efficiently a hydrocarbon burns. (a) Draw the shortened structural formula of 2-methylbutane (b) Draw the full structural formula of 2-methylhexane. (c) Predict the octane number of 2-methylheptane. 9. Dienes are a homologous series of hydrocarbons that contain two double bonds per molecule. (a) Suggest a general formula for the dienes. (b) Write the molecular formula for buta-1,3-diene. (c) Draw the full structural formula of an isomer of buta-1,3-diene which contains only one double bond per molecule. (d) Draw the full structural formula for the product of the complete reaction of penta-1,3-diene with bromine. 10. In industry, ethanol (an alcohol) can be produced from ethane as shown below. (a) Name the type of reaction taking place. (b) Draw the full structural formula for the product of the following reaction.