Energy and Reactions Review Name Period ______ The role of
advertisement

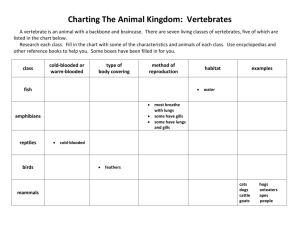
Energy and Reactions Review Name ________________________ Period ______ The role of energy in chemical reactions is very important in life. In the cells of our body, countless numbers of reactions are allowing our bodies to do everything such as movement, thinking, hearing, etc. For this class there are a couple basic concepts we need to understand regarding reactions. Reactions can be classified as either: Endothermic – reaction takes in energy as it progresses (that means the environment temperature decreases) Exothermic – reaction releases energy as it progresses (that means the environment temperature increases) With exothermic reactions, stored energy in the chemical bonds of a molecule is released. One key example is the breakdown of glucose into carbon dioxide. This reaction happens in our cells and allows us to use the energy released to produce an important energy molecule cells need called ATP. Energy stored in a molecule is termed potential energy. When that energy is released as things like heat or light, we call it kinetic energy. Most reactions do not happen spontaneously meaning they need to have a little “nudge” to get them going. Think about a match, once it is burning it will continue to do so until the reaction runs out of fuel. The energy needed to start a reaction is called the activation energy. Parts of a Reaction The reactants are the substances that are present before the chemical change takes place. They are the things that are present at the starting point. By convention, the chemical symbols for the reactants are written on the left hand side of the chemical reaction equation. The products are the substances that are formed during the chemical change. They are the things that are present at the end. By convention, the chemical symbols for the products are written on the right hand side of the chemical reaction equation. A reaction arrow is the symbol that signifies the actual change. A reaction arrow separates the reactants from the products. While a typical chemical reaction equation will include much more information, they all have the substances present before and after the chemical change with a reaction arrow to separate them. C 3H 8 + 5O2 propane + oxygen Reactants yields -------- 4H2O + 3CO2 water + carbon dioxide Products-------------------- + + energy energy ENERGY IS NOT A PRODUCT 1. Label the reactants and products of the above reactions Energy is not matter..therefore is not a product 2. How many individual carbon-containing molecules are there in the reactants side? Only 1 molecule (C3H8) 3. How many individual carbon-containing molecules are there in the product side? There are 3 CO2 molecules 4. Is this exothermic or endothermic? Since energy is on the product side, it is exothermic 5. This reaction is what happens in a propane barbeque grill. What supplies the activation energy for this reaction? The spark (created from friction) that ignites the gas 6. What would happen to this reaction if oxygen were removed from the environment? It would not happen 1 Energy and Reactions Review Name ________________________ Period ______ 7. Is cellular respiration (the breakdown of glucose to CO2 and H2O) exothermic or endothermic? What happens to the heat that is generated? What other energy molecule is created during this process? Is given off (usually water absorbs it and is removed via sweat. ATP Glow Stick Time! Observe the glow stick as it is moved between the hot and cold environments. What changes do you observe? Relate these changes to what we have discussed about the effects of energy on molecular movement (think states of matter and energy within the matter) Cold Room Temp Hot More energy means more motion of the atoms. Increased motion increases the likelihood of collisions and reactions A common theme in this class is the role of energy in life. Temperature is one of these critical factors as it is closely involved in the chemical reactions we rely on to do everything. What role does temperature play in living systems? All plants and animals are adapted to survive within a temperature range (which can be different for different organisms). This dictates whether an animal hibernates, when plants start to germinate, and what climate organisms “thrive in” and even for some (like Siamese cats) the color of their extremities. It also dictates migration cycles and even reproduction cycles. Temperature differences in the ocean create ocean currents (and therefore move heat from the tropics to the poles and cold from the poles to the tropics In the history of evolution of life on this planet, the first vertebrates were termed exothermic (cold-blooded). Cold-blooded creatures take on the temperature of their surroundings. They are hot when their environment is hot and cold when their environment is cold. In hot environments, cold-blooded animals can have blood that is much warmer than warm-blooded animals. The first vertebrates to appear were the ancestors of fish. Why would it be easier for fish to be cold blooded vs. land vertebrates? Think about living conditions! Because water has high heat capacity the fluctuation in temperature is much less than “land” conditions. Cold-blooded animals are much more active in warm environments and are very sluggish in cold environments. This is because their muscle activity depends on chemical reactions which run quickly when it is hot and slowly when it is cold. A cold-blooded animal can convert much more of its food into body mass compared with a warm- 2 Energy and Reactions Review Name ________________________ Period ______ blooded animal. The first land vertebrates were the ancestors of modern amphibians (frogs, toads, etc.) and reptiles (lizards, snakes.) What adaptations do you know that amphibians and reptiles employ to regulate body temperature? Amphibians have moist skin, acts as an evaporative cooling system (amphibians). This is why they must live in moist conditions Bull frogs can actually control amt of mucus they secrete. Reptiles have dry skin which reduces heat loss , an expandable rib cage (allows for better ventilation). Diving reptiles actually have circulatory shunts to “focus” circulation (and warmth) in the central body. Warm-blooded creatures, like mammals and birds, try to keep the inside of their bodies at a constant temperature. They do this by generating their own heat when they are in a cooler environment, and by cooling themselves when they are in a hotter environment. To generate heat, warm-blooded animals convert the food that they eat into energy. This places a much greater importance on regular consumption of foods. Only a small amount of the food that a warm-blooded animal eats is converted into actual body mass. Which mammal would need to dedicate a greater percentage of energy gained from food for body heat regulation, a cow or a mouse? Explain. Relate to differences in diet. Body heat is based on surface area to volume ratio. Since mice have much higher PERCENT surface area (as compared to the volume of their body) they will lose heat faster, and therefore need more food (per unit of mass) to stay warm Why do pet snakes only need to be fed rarely while pet mice need to be fed daily? Cold blooded, can slow their metabolism In the desert, reptiles have a much easier time existing. Why would they find it easier than warm blooded organisms to exist in hot climates? They can use the sun (or warmth taken into the sand by the sun to warm themselves (not requiring food energy to be warm). They burrow, and by controlling their metabolism by external sources, need less food to maintain their temperature. 3