Ch27Cells and Batteries 08
advertisement
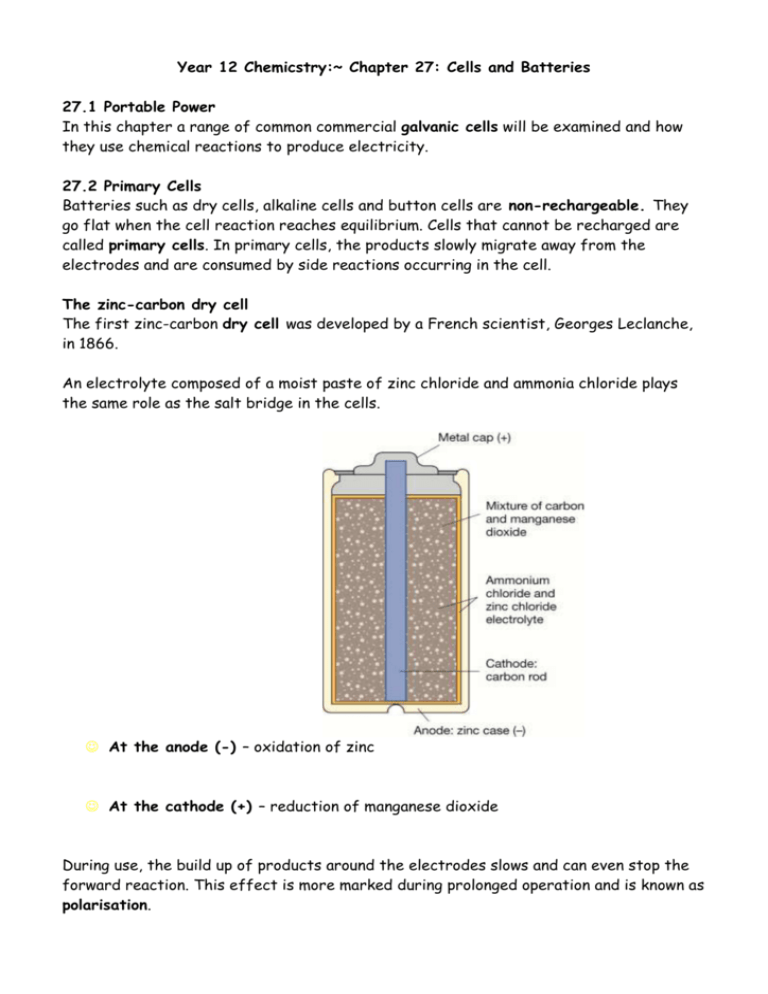
Year 12 Chemicstry:~ Chapter 27: Cells and Batteries 27.1 Portable Power In this chapter a range of common commercial galvanic cells will be examined and how they use chemical reactions to produce electricity. 27.2 Primary Cells Batteries such as dry cells, alkaline cells and button cells are non-rechargeable. They go flat when the cell reaction reaches equilibrium. Cells that cannot be recharged are called primary cells. In primary cells, the products slowly migrate away from the electrodes and are consumed by side reactions occurring in the cell. The zinc-carbon dry cell The first zinc-carbon dry cell was developed by a French scientist, Georges Leclanche, in 1866. An electrolyte composed of a moist paste of zinc chloride and ammonia chloride plays the same role as the salt bridge in the cells. At the anode (-) – oxidation of zinc At the cathode (+) – reduction of manganese dioxide During use, the build up of products around the electrodes slows and can even stop the forward reaction. This effect is more marked during prolonged operation and is known as polarisation. A new cell produces about 1.5 volts, but this diminishes significantly during use. To maintain a net forward reaction, the soluble reactions products must migrate away from the electrodes. Zinc-carbon dry cells now tend to be restricted to applications that require a cheap portable source of energy for infrequent low-drain use. Alkaline Cell The alkaline cell was invented in the late 1800s and appeared on the market in the late 1960s. The alkaline cell is optimised for performance and longevity. At the anode (-) – zinc powder is oxidised Zn2+ reacts immediately with OH- ions in the electrolyte At the cathode (+) – manganese dioxide is reduced Alkaline cells are more expensive however have five times the life as do dry cells. These batteries are good for high currents and intermittent use. Use: Button cell There are two main types: silver-zinc cells and lithium cells. Both are relatively expensive because of their small size and the materials used to make them. Lithium button cells produce about 3 volts during discharge and silver-zinc cells give an almost constant 1.6 volts. Lithium button cell At the anode (-) At the cathode (+) Uses: heart pacemaker: variant on the lithium cell by adding iodine. Example: The overall reaction that occurs in the silver-zinc button cell is: Zn(s) + Ag2O(s) + H2O(l) Zn(OH)2(s) + 2Ag(s) Write equations for the anode and cathode: Anode: Cathode: (remember that zinc ions react with hydroxide from the electrolyte. Example: The following reactions occur in the lithium button cell: Li(s) Li+(aq) + eMnO2(aq) + Li+(aq) + e- LiMnO2(s) a) Which reaction occurs at the negative electrode? b) Write an overall equation for the reaction occurring in the lithium cell. 27.3 Rechargeable and Non-Rechargeable Cells Rechargeable cells, such as lithium ion cells, are known as secondary cells or accumulators. To recharge a cell, the products of the reaction must be converted back into the original reactants. A source of electrical energy, which has a potential difference greater than the potential difference of the cell. In order for it to be possible to regenerate the reactants, the products formed in the cell during discharge must remain in contact with the electrodes in a convertible form. Car Batteries/ Lead-Acid Battery Lead-acid batteries are the most widely used type to storage cell. - relatively cheap and reliable; - provide high currents; - long lifetime. The battery is used to start the car then the alternator takes over. The alternator can also provide electrical energy to recharge the battery. A lead-acid battery contains six separate cells connected together in series. Contact between the electrodes is prevented by the presence of a porous separator. The positive electrodes consist of a lea grid packed with lead(IV)oxide (PbO2), while the negative electrodes consist of a lead grid packed with powdered lead. A solution of 4M sulfuric acid acts as the electrolyte. Each cell has a potential difference of just over 2 volts. A car battery has six of these cells connected in series, giving a total potential difference of about 12 volts. At the anode (-) At the cathode (+) The Overall equation: The product of both electrode reactions, lead (II) sulfate, forms as a solid on the surface of the electrodes. This feature enables the battery to be recharged. The recharge equation is: Nickel-bases cells and batteries A Swedish engineer Waldemar Jungner in 1899 invented the nickel-cadmium cell. Although still widely used today, it is slowly being overtaken by its better performing and more environmentally friendly relative the nickel-metal hydride cells. The cell consists of a coiled sandwich of anode, porous separator and cathode immersed in a concentrated potassium hydroxide electrolyte. At the anode (-): In a nickel-cadmium cell, oxidation of cadmium generates electrons and the Cd2+ ions react immediately with OH- ions. At the anode (-): In a nickel-metal hydride cell, the negative electrode is made of a special hydrogen-absorbing metal alloy (MH). The absorbed hydrogen reacts with OH-. At the cathode (+): nickel ions are reduced to nickel hydroxide Most brands of nickel-cadmium cells are capable of 1000 discharge-recharge cycles. QUESTIONS: 3 (summary notes) 5, 6, 8, 14, 16 & 22. Year 12 Chemistry:~ Chapter 27: Fuel cells 27.4 Fuel Cells Cells can be constructed in which the reactants are supplied continuously, allowing constant production of electrical energy. Such devices are called fuel cells. Fuel cells transform chemical energy directly into electrical energy, enabling efficient use of the energy released by spontaneous redox reactions. Fuel cells are up to 80% efficient, compared with 30 – 40% for thermal power stations. In addition, modern designs for fuel cells employ the waste heat that they produce to make steam. This steam can be used for heating or to operate a turbine, thus increasing the efficiency. The basic principles behind the operation of a fuel cell were discovered in 1839. Fuel cells were the main on-board power supply units used during the Apollo Program. An explosion in a fuel cell was responsible for the failure of the Apollo 13 mission to the moon. The fuel cell used in the Apollo program used pure oxygen and hydrogen gas as reactants. They are commonly referred to as alkaline fuel cells because potassium hydroxide is used as the electrolyte. At the anode (-) – hydrogen gas reacts with hydroxide from the electrolyte At the cathode (+) Overall reaction: Each cell produces about 1 volt and the only by-products are water and heat. The electrodes are very important as they act as a catalyst. Advances in fuel-cell technology have led to the development of the acid fuel cell, which uses concentrated phosphoric acid as the electrolyte and doesn’t need pure hydrogen. Over 400 phosphoric acid fuel cells are in use around the world and there is one in Sydney. Fuel cells are named according to the electrolyte and catalysts used in them. Transport In Perth, buses using hydrogen-powered fuel cells have been in operation for a number of years. This use of fuel cells improves fuel efficiency and reduces greenhouse gases and other emissions as well as our reliance on oil. Electricity generation Stationary fuel cell systems are used to generate electricity for domestic, commercial and industrial purposes. Small appliances A methanol-powered fuel cell has been developed for use with portable electronic equipment such as mobile telephones and lap-top computers. Advantages and disadvantages Advantages Disadvantage QUESTIONS: 9, 10, 19, 21 & 23.