Solid Oxide Fuel Cell (SOFC)
advertisement

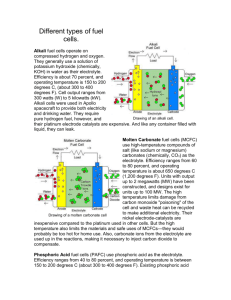
Solid Oxide Fuel Cell (SOFC) The Solid Oxide Fuel Cell (SOFC) is currently the highest-temperature fuel cell in development and can be operated over a wide temperature range from 700ºC–1000ºC allowing a number of fuels to be used. To operate at such high temperatures, the electrolyte is a thin, solid ceramic material (solid oxide) that is conductive to oxygen ions (O2-). 1. HISTORY Research into solid oxide technology began to accelerate in late 1950’s at the Central Technical Institute in The Hague, Netherlands, Consolidation Coal Company, in Pennsylvania, and General Electric, in Schenectady, New York. A 1959 discussion of fuel cells noted that problems with solid electrolytes included relatively high internal electrical resistance, melting, and short-circuiting due to semi conductivity. It seems that many researchers began to believe that molten carbonate fuel cells showed more short-term promise. Not all gave up on solid oxide, however. The promise of a high-temperature cell that would be tolerant of carbon monoxide and use a stable solid electrolyte continued to draw modest attention. Researchers at Westinghouse, for example, experimented with a cell using zirconium oxide and calcium oxide in 1962. More recently, climbing energy prices and advances in materials technology have reinvigorated work on SOFCs, and a recent report noted about 40 companies working on these fuel cells. 2. TECHNOLOGY A solid oxide fuel cell (SOFC) uses a hard ceramic electrolyte instead of a liquid and operates at temperatures up to 1,000 degrees C (about 1,800 degrees F). A mixture of zirconium oxide and calcium oxide form a crystal lattice, though other oxide combinations have also been used as electrolytes. The solid electrolyte is coated on both sides with specialized porous electrode materials. At this high operating temperature, oxygen ions (with a negative charge) migrate through the crystal lattice. When a fuel gas containing hydrogen is passed over the anode, a flow of negatively charged oxygen ions moves across the electrolyte to oxidize the fuel. The oxygen is supplied, usually from air, at the cathode. Electrons generated at the anode travel through an external load to the cathode, completing the circuit and supplying electric power along the way. Generating efficiencies can range up to about 60 percent. SOFC Stacking A 40-cell SOFC stack with 16-cm diameter cells achieves 1.40 kW at 0.428 A/cm2 with 80% fuel utilization and an average cell voltage of 0.673 V Four common SOFC stack configurations have been proposed and fabricated - the flat plate design, the seal less tubular design, the segmented-cell-in-series design and the monolithic design. • Flat-plate design The simplest way to envisage a SOFC as a single plate cell is by stacking components on top of each other. This design offers simple cell geometry and multiple fabrication options such as tape calendaring or tape casting. • Seal less tubular design The seal less tubular design consists of the cell components configured as thin layers on a tubular support closed at one end. The problems encountered previously with gas seals are now eliminated with this array, although the cell is still hampered by the limited gas flow through the tube and relatively long current path. • Segmented cell-in-a-series This design consists of segmented cells connected in electrical and gas flow series. The cells are either arranged as a thin banded structure on a porous support or filleted one into the other to form a tubular selfsupporting structure. The major problem is low gas flow, which results from the thick support tube and the robust seals required at the ends of the tube. • Monolithic The monolithic design is the newest SOFC stack concept. It consists of many cells fabricated as a single unit. The design has the potential to achieve high power density because of its compact and lightweight structure. Nevertheless, they are difficult to manufacture and have a higher likelihood of cracking during operation due to the expansion mismatches of the materials. In Siemens Westinghouse's stack design, the tubular cells have a vertical orientation with the closed end down. The Siemens Westinghouse stack has progressed most recently from successful tests of 25 kW modules that used cells 50 cm in active length to the present prototype module using the 150 cm. cells, which are the cell size that will be carried forward in our commercial systems. A standard module of 1152 cells can produce up to 200 kW dc but is given a nominal rating of 100 kW ac. The prototype cells are arranged into 3 x 8 cell bundles, and the bundles into bundle rows. Between each bundle row is placed an in-stack reformer. The reformer is radiantly heated by the adjacent rows of cells. The outer container as shown applies to an atmospheric pressure unit. 3. OPERATION An SOFC consists of two porous electrodes separated by a dense oxygen-ion conducting Electrolyte. Oxidant is reduced at the cathode side and fuel is oxidized at the anode. The difference in oxygen activity of the two gases at the electrodes provides a driving force for motion of oxide ions in the electrolyte. Oxide ions formed by dissociation of oxygen at the cathode under electron consumption migrate through electrolyte to the anode where they react with hydrogen to form water and release electron. (The oxygen is dissociated at the cathode into O2-, then the cat ion travels through the high-temperature ionic conducting electrolyte and mixes with the hydrogen at the anode form water and release electrons.) The main characteristic of SOFCs is therefore their high operating temperature (from 700°C to 1000°C), which is needed to attain an adequate level of ionic conductivity in the ceramic electrolyte. There is a dual advantage to this high temperature. Firstly, it allows the direct use of hydrocarbons, in the first instance natural gas, which can easily be reformed without using catalysts based on noble metals. Secondly, it provides a source of intense heat that can be used without difficulty in a cogeneration system, either with or without a gas turbine: in this way, the overall efficiency increases reaching up to 80%. But there is a drawback: the heating up process is long, and therefore not appropriate for short, repetitive cycles. 1. Electrolyte SOFC is based on the concept of an oxygen-ion conducting electrolyte where oxide ions (O2-) migrate from cathode to anode and react with the fuel to generate electricity. Oxide materials with fluorite crystal structure such as yttria-stabilized zirconia (YSZ), rare earth-doped ceria and rare earth-doped bismuth oxide have been widely investigated as electrolytes for fuel cell. Zirconia doped with 8-10mole% yttria (YSZ) is the most wideused electrolyte for SOFC because it conducts only oxygen ions over a wide range of oxygen partial pressure. 2. Anode (Fuel electrode) The electrode must be stable in the reducing environment of the fuel, should be electronically conducting and must have sufficient porosity to allow transport of the products of fuel oxidation away from electrolyteelectrode interface. In this region the fuel oxidation reaction is: O2- (s) + H2 (g) H2O (g) + 2e3. Cathode (Air electrode) The cathode is the site for the electrochemical reduction of oxidant. Therefore, the cathode material must be chemically and thermally stable in the oxidizing atmosphere. In addition, a potential cathode material should be reasonably compatible with other cell components. 4. Sealing materials Method of sealing the ceramic components to obtain gas-tightness is a major issue of SOFC. Glass ceramics are used as sealant, although migration of the silica component can still be a problem on anode and cathode. 5. Interconnecting materials (External Circuit) As the name implies, the interconnecting material connects anode of one cell with cathode of another cell so that voltage output could be enhanced. Doped lanthanum chromite (LaCrO3) has been used as the interconnecting material since the 1970s. 6. Catalyst (Nickel, Copper) The catalyst within the anode promotes release of free electrons from the cells fuel source. The catalyst within the cathode promotes the generation of oxygen ions from the cells oxygen source. When separated by an electrolyte membrane layer that allows passage of ions while limiting electron flow, fuel cell anodes and cathodes work together to release free electrons that are available to do work as part of an external circuit. 7. Hydrogen feed SOFC devices can internally reform some fuels to deliver hydrogen fuel, and they can be fabricated in a variety of shapes and form factors. 8. Oxygen Feed At the cathode, the oxygen provided by the air or oxidant feed reacts with electrons in-bound from the external circuit and H+ protons coming through the electrolyte to form water. The water is expelled, along with any other compounds in the oxidant feed stream out through the cathode exhaust. 9. Exhaust The SOFC exhaust exits the generator module at a temperature of between 800 degrees C and 850 degrees C and in atmospheric pressure systems is passed through the exhaust gas heat recovery train. This heat can be adapted to generate process heat or hot water for a combined heat and power application 4. ADVANTAGES 1. SOFCs can achieve electrical efficiency of up to 50% using natural gas and can also achieve this performance with other hydrocarbon fuels such as liquefied petroleum gas. As a result of its high operating temperatures, SOFCs can also be combined with heat recovery technologies such as heat exchangers to create a total system efficiency of up to 85%. 2. SOFC is the most inherently fuel flexible of the fuel cell types 3. Broad product range capability SOFC technology can support distributed generation (DG) products such as generators and combined heat and power units in the capacity range from small residential to large industrial sizes, as well as automotive applications such as auxiliary power units. In DG applications, the low air and noise emissions of SOFC-based systems allow for ease of sitting and permitting. The high operating temperature also results in high-grade exhaust heat, which can be utilised in a wide range of cogeneration applications. 4.High reliability SOFCs are made from commonly available ceramic materials. SOFC technology has no moving parts or corrosive liquid electrolytes. They are expected to lead to electricity generation systems that are highly reliable and require low maintenance 5. Solid Electrolyte One of the big advantages of the SOFC over the MCFC is that the electrolyte is a solid. This means that no pumps are required to circulate hot electrolyte, moreover there is no leakage problem with the electrolyte 5. LIMITATIONS 1.Though the Solid Electrolyte cannot leak, it can break and provide a severe problem 2. Higher stack temperatures demand exotic materials, which add to manufacturing costs. Heat also presents a challenge for longevity and reliability because of increased material oxidation and stress 3. Unfortunately, the dominant SOFC developers aim at stationary applications Such ceramic solutions are indeed heavy, sluggish, expensive and fragile and must be operated at high temperatures. But totally different SOFCs are presently developed for mobile applications. 6. APPLICATION 1.SOFC micro-power plants take away the dependence and limitations of the electric distribution grid, in a remote standalone package that can also provide heat for the home. This lets the homeowner live just about anywhere, in the mountains or deep woods, in the desert or on an island. 2.Solid oxide fuel cell (SOFC) power systems for commercial and industrial applications are designed to provide clean, highly efficient power for on-site grid-support, grid-back-up or grid-independent electrical generation needs. 3.SOFC can be used as uninterruptible power supplies (UPS), especially in military application as back up power 4.For high-priority carrier, provider, corporate or government networks SOFC provides high grade power 7. Block Diagrams (Various Fuel cells) 4. Future Scope (SOFC) Scientists are currently exploring the potential for developing lower-temperature SOFCs operating at or below 800°C that have fewer durability problems and cost less. Lower-temperature SOFCs produce less electrical power, however, and stack materials that will function in this lower temperature range have not been identified. 8. Some other useful links http://www.eere.energy.gov/hydrogenandfuelcells/fuelcells/fc_types.html http://www.personal.psu.edu/users/a/b/abs193/activities/cause_2003/renewable_energy_presentation.pdf http://www.benwiens.com/energy4.html http://www.fctec.com/fctec_types_sofc.asp http://www.cea.fr/gb/publications/Clefs44/an-clefs44/clefs4453a.html http://www0.nsc.co.jp/shinnihon_english/kenkyusho/contenthtml/n92/n9216.pdf http://www.iit.edu/~smart/garrear/fuelcells.htm