南京晓庄学院Inorganic Chemistry课程考试试卷 A B
advertisement
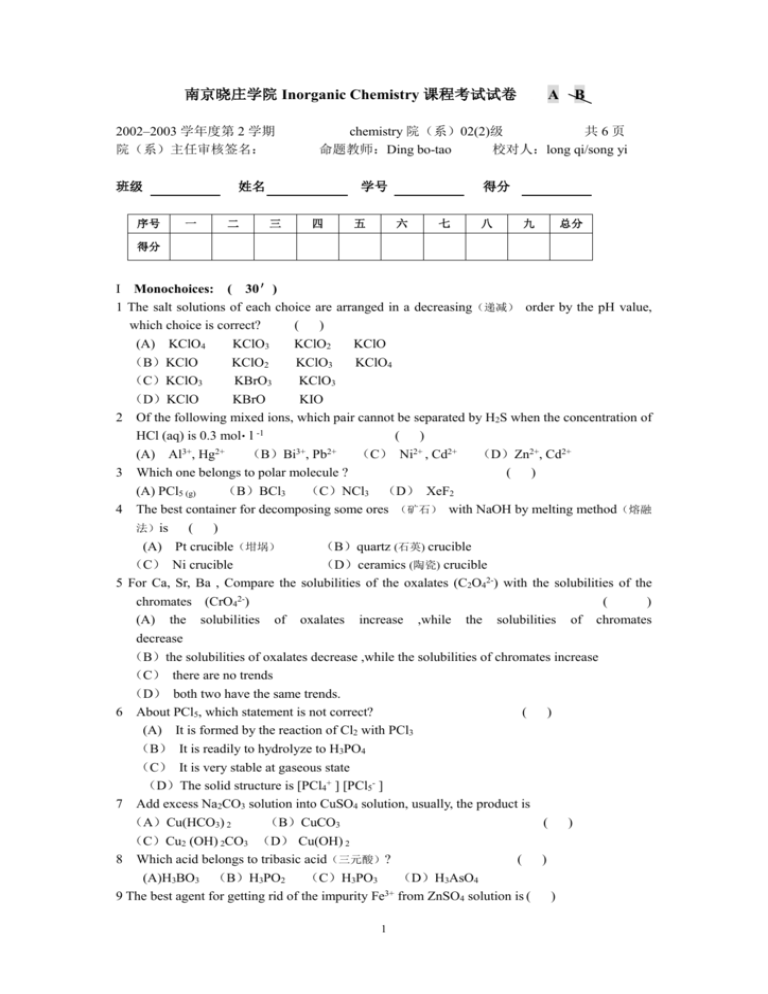
南京晓庄学院 Inorganic Chemistry 课程考试试卷 2002–2003 学年度第 2 学期 院(系)主任审核签名: 班级 序号 二 学号 三 B chemistry 院(系)02(2)级 共6页 命题教师:Ding bo-tao 校对人:long qi/song yi 姓名 一 A 四 五 得分 六 七 八 九 总分 得分 I Monochoices: ( 30') 1 The salt solutions of each choice are arranged in a decreasing(递减) order by the pH value, which choice is correct? ( ) (A) KClO4 KClO3 KClO2 KClO (B)KClO KClO2 KClO3 KClO4 (C)KClO3 KBrO3 KClO3 (D)KClO KBrO KIO 2 Of the following mixed ions, which pair cannot be separated by H2S when the concentration of HCl (aq) is 0.3 mol• l -1 ( ) 3+ 2+ 3+ 2+ (A) Al , Hg (B)Bi , Pb (C) Ni2+ , Cd2+ (D)Zn2+, Cd2+ 3 Which one belongs to polar molecule ? ( ) (A) PCl5 (g) (B)BCl3 (C)NCl3 (D) XeF2 4 The best container for decomposing some ores (矿石) with NaOH by melting method(熔融 法)is ( ) (A) Pt crucible(坩埚) (B)quartz (石英) crucible (C) Ni crucible (D)ceramics (陶瓷) crucible 5 For Ca, Sr, Ba , Compare the solubilities of the oxalates (C2O42-) with the solubilities of the chromates (CrO42-) ( ) (A) the solubilities of oxalates increase ,while the solubilities of chromates decrease (B)the solubilities of oxalates decrease ,while the solubilities of chromates increase (C) there are no trends (D) both two have the same trends. 6 About PCl5, which statement is not correct? ( ) (A) It is formed by the reaction of Cl2 with PCl3 (B) It is readily to hydrolyze to H3PO4 (C) It is very stable at gaseous state (D)The solid structure is [PCl4+ ] [PCl5- ] 7 Add excess Na2CO3 solution into CuSO4 solution, usually, the product is (A)Cu(HCO3) 2 (B)CuCO3 ( ) (C)Cu2 (OH) 2CO3 (D) Cu(OH) 2 8 Which acid belongs to tribasic acid(三元酸)? ( ) (A)H3BO3 (B)H3PO2 (C)H3PO3 (D)H3AsO4 9 The best agent for getting rid of the impurity Fe3+ from ZnSO4 solution is ( ) 1 (A) NaOH (B) Na2CO3 (C) ZnCO3 (D) Zn 10 A and B are in ds-block elements. Add appropriate amount of (适量的) KI solution into a sulphate solution of A and a chloride solution of B , respectively(分别地),in the sulphate solution of A, forming an iodide precipitate of A and I2 , but in the chloride solution of B, only giving an iodide precipitate of B ,which can dissolve in excess KI solution to form a complex. Then the sulphate solution of A and the chloride solution of B are (A) ZnSO4 Hg2Cl2 (B)CuSO4 HgCl2 ( ) (C)CdSO4 HgCl2 (D)Ag2SO4 Hg2Cl2 11 Which pair of the elements has the most similar properties? (A) Mg and Al (B) Zr and Hf ( ) (C)Ag and Au (D)Fe and Co 12 Which ion doesn’t exist in aqueous solution? ( ) 3+ 4+ (A) [Ti(H2O) 6] (B)[Ti(H2O) 6] 2+ (C) [Ti(OH) 2 (H2O) 4] (D)[Ti(O2) OH(H2O) 4] + 13 The different chemical property of Al (III) with Cr (III) is ( ) (A) the ability of forming complex (B) the ability of forming double salts (C)the extent of hydrolysis (D)the amphoteric property 14 When MnO4 reacts with I in a concentrated and strongly basic solution ,the most possible products are ( ) (A) Mn (s) and I2 (B)MnO4 and IO3 (C)MnO2, O2 and IO- (D)Mn2+ and I2 15 Which compound belongs to heteropolyacids (杂多酸)? ( ) (A) Na3[P(W12O40)] (B)KCr(SO4)2 •12 H2O (C)Na4Mo 7O 23 (D)Fe2 (CO) 9 16 Which hydroxide doesn’t change its colour when stands in the air after being prepared ? ( ) (A) Fe(OH) 2 (B)Mn(OH) 2 (C)Co(OH) 2 (D)Ni(OH) 2 17 Which oxide doesn’t produce O2 when heated with concentrated H2SO4? (A) CrO3 B)MnO2 (C)PbO2 (D)V2O5 ( ) 18 Which hydroxide has the smallest solubility? ( ) (A) Ba(OH) 2 (B) La(OH) 3 (C)Lu(OH) 3 (D) Ce(OH) 4 II Fill in the following blanks ( 1 To separate BaSO4 from PbSO4 , 2 of IA and 30') or could be added into the mixture. of II A can combine with N2 directly to form nitride. 3 The weakest acid among H2S, H2Se, H2Te, H2SO4, H2SeO3 is 4 The three pairs of the elements with the diagonal relationship(对角线关系)in the periodic table are ; ; . 5 Compare the extent of hydrolysis between the following ions (a)Among Fe3+ ,Cu2+ ,Ti4+ ,Ag+ , the strongest is ,the weakest is 2 (b) Among S2- , HPO42-, NO3-, SO32-, the strongest is ,the weakest is 6 Among the acids H6TeO6 , H2SeO3 , HReO4 , HClO2 , the strongest acid is weakest acid is 7 Among the four compounds: SnCl2, Bi(OH) 3, Mg3N3, (NH4) 2SO4, gas and forms a precipitate in water. gives out a 8 Among the hydroxides, Ni(OH)2 , Cu(OH)2 , Ga(OH)3 , Mn(OH)2 , are amphoteric 9 Among and the hydroxides, Zn(OH)2 Fe(OH)3 can dissolve in NH3 (aq)to form complexes. Fe(OH)2 ,the Cd(OH)2 and Pb(OH)2, 10 Excess KI solution is added into a solution of Hg(II).After NaOH solution is added gradually (逐渐地)till the solution becomes strongly basic, a little ammonium solution (NH4+) is added into this strongly basic solution, and a red-brown precipitate forms quickly. Write the chemical equations 11 At room temperature, excess NaOH is added into four solutions containing Ag+, Hg22+, Zn2+,Cd2+ respectively( 分 别 地 ).The main products of each reaction is ; ; ; 12 Appropriate amount of KI is added into two solutions of CuSO4 and HgCl2 respectively, the product of each reaction is ; .Treat (处理) the latter product with excess KI solution, and it can dissolve because of forming 13 The reduction potential diagram of Manganese is as follows: MnO4| 0.564V MnO42- 1.69V | among the above ions, 2.26V MnO2 0.95V Mn3+ | 1.51V 1.23V Mn2+ -1.18V Mn | | 1.51V and are unstable and can disproportionate. 14 K 2Cr 2O 7 solution reacts with BaCl 2, KOH, hot HCl (conc.) and respectively, then the products are ; ; ; 15 H2O2(ether,乙醚) Na2CO3 solution is added into FeCl3 and FeSO4 respectively, the products are ; . (the colors of all the precipitates should be written). Put the latter in the air. It converts (变成) to III calculations ( 20') 1 (5’) Silver is used as catalyst in industry. It is produced by the following decomposition: 3 AgNO3 (s) = Ag(s) + NO2 (g) + 1/2 O2 (g) Evaluate(估计)the lowest decomposing temperature for the decomposition by calculation. Given data: for for for for θ -1 AgNO3(s) S mθ=140 J• mol-1• K-1 f H m = -123.14 kJ• mol θ -1 NO2 (g) S mθ=240.6 J•mol-1• K-1 f H m = 35.15 kJ •mol Ag(s) S mθ= 42.68 J• mol-1• K-1 O2 (g) S mθ= 205 J• mol-1 •K-1 2 (10’) For a voltaic cell: Cu|Cu 2+ (1.0mol•l-1) ‖AuCl4 - (0.10 mol•l-1) Cl- (0.10 mol•l-1)|Au ФθAu3+/Au = 1.5 0 V ФθAuCl4-/Au = 1.0 0 V ФθCu2+/Cu = 0.34 V (1) write the half reactions and the overall reaction. (2) calculate the cell potential Eθ for the voltaic cell (3) calculate the Kθ for the overall reaction (4) calculate the Kf for the AuCl4- 4 3 (5’)Calculate and explain whether CdS can precipitate by bubbling H2S gas through the solution of Cd(CN) 42given data: Kf Cd(CN) 42- = 8×1018 Ksp CdS = 8×10-27 Ka1 Ka2 H2S = 9.2×10-22 KaHCN = 6.2×10-10 IV Answer the following questions briefly. 1 (5’)Atom A has one electron on the subshell of n = 5, l =0 and its subshell of n = 4, l = 2 is full. Atom B is in the same period with A. If the simple ions of A and B are mixed together, then a yellow precipitate AB will form. (1) Write the electronic configuration of A and B,and show the valence electrons. (2) Which period and which group are A and B in ,respectively? Is A a metal or a nonmetal? Is B a metal or nonmetal l? (3) Write the formula(化学式) of AB. 2 (5’)Is it reasonable(合理的)that a solution contains Ag+, K+, S2O32-, Sn2+ simultaneously(同时)? Write your reasons. 5 3 (5’) Draw a diagram to show how to separate and test the three ions:Zn2+,Mg2+,Ag+ 4 (5’)Given H 2SO 4,CaO and NaHSO 3,design some reactions for getting rid of chromium from waste water containing Cr(VI)and Cr(III).Write all the chemical equations. 6