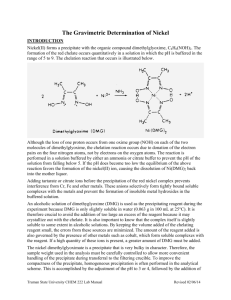
Exeriment 2: Gravimetric Determination of Iron as Fe2O3
advertisement
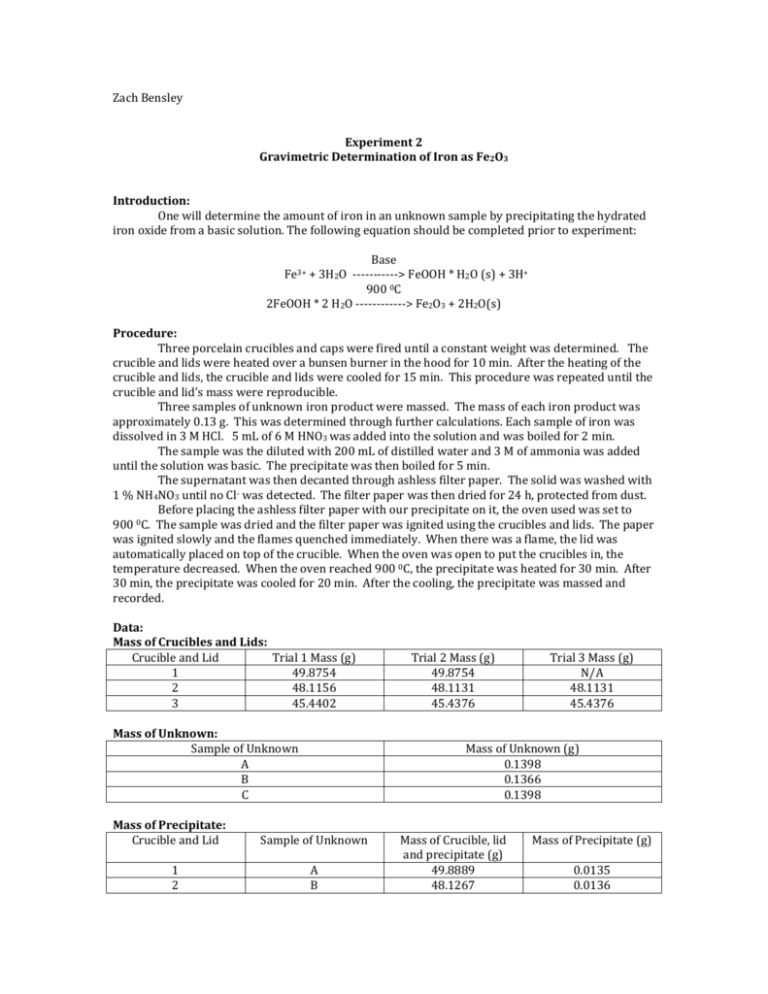
Zach Bensley Experiment 2 Gravimetric Determination of Iron as Fe2O3 Introduction: One will determine the amount of iron in an unknown sample by precipitating the hydrated iron oxide from a basic solution. The following equation should be completed prior to experiment: Base Fe3+ + 3H2O -----------> FeOOH * H2O (s) + 3H+ 900 0C 2FeOOH * 2 H2O ------------> Fe2O3 + 2H2O(s) Procedure: Three porcelain crucibles and caps were fired until a constant weight was determined. The crucible and lids were heated over a bunsen burner in the hood for 10 min. After the heating of the crucible and lids, the crucible and lids were cooled for 15 min. This procedure was repeated until the crucible and lid’s mass were reproducible. Three samples of unknown iron product were massed. The mass of each iron product was approximately 0.13 g. This was determined through further calculations. Each sample of iron was dissolved in 3 M HCl. 5 mL of 6 M HNO3 was added into the solution and was boiled for 2 min. The sample was the diluted with 200 mL of distilled water and 3 M of ammonia was added until the solution was basic. The precipitate was then boiled for 5 min. The supernatant was then decanted through ashless filter paper. The solid was washed with 1 % NH4NO3 until no Cl- was detected. The filter paper was then dried for 24 h, protected from dust. Before placing the ashless filter paper with our precipitate on it, the oven used was set to 900 0C. The sample was dried and the filter paper was ignited using the crucibles and lids. The paper was ignited slowly and the flames quenched immediately. When there was a flame, the lid was automatically placed on top of the crucible. When the oven was open to put the crucibles in, the temperature decreased. When the oven reached 900 0C, the precipitate was heated for 30 min. After 30 min, the precipitate was cooled for 20 min. After the cooling, the precipitate was massed and recorded. Data: Mass of Crucibles and Lids: Crucible and Lid Trial 1 Mass (g) 1 49.8754 2 48.1156 3 45.4402 Mass of Unknown: Sample of Unknown A B C Mass of Precipitate: Crucible and Lid 1 2 Trial 2 Mass (g) 49.8754 48.1131 45.4376 Trial 3 Mass (g) N/A 48.1131 45.4376 Mass of Unknown (g) 0.1398 0.1366 0.1398 Sample of Unknown A B Mass of Crucible, lid and precipitate (g) 49.8889 48.1267 Mass of Precipitate (g) 0.0135 0.0136 3 C 45.4500 0.0125 Mass of Solid and Final Precipitate: Trial 1 2 3 Initial Solid Mass (g) 0.1398 0.1366 0.1398 Precipitate Mass (g) 0.0135 0.0136 0.0125 Calculated Data: Theoretical Yield (g) Percent Yield Percent Recovery Trial 1 0.0314 57.2% 42.8% Trial 2 0.0307 55.7% 44.3% Calculations: Mass of Precipitate: Mass of Precipitate: Mass final-Mass initial= Mass of Precipitate Mass final – Mass initial 49.8887 g – 49.8754 g = 0.0135 g Mass final= Mass of Crucible, lid and solid Mass initial= Mass of crucible and lid Theoretical Yield M initalsolid * 0.75 * 1molFeNH4 (SO4 ) 2 1molFe 1molFe2 0 3 159.69gFe2 0 3 * * 265.937gFeNH4 (SO4 ) 2 1molFeNH4 (SO4 ) 2 2molFe 1molFe2 0 3 Percent Error: 0.0135 0.0314 * 100% = 57% 0.0314 Percent Recovery: Theoretical Yield For solving the theoretical yield, Minitalsolid= 0.1398 g Theoretical yield = 0.0314 g Percent Error: Actual Expected *100% = Percent Error Expected Pr ecipitateMass *100% TheoreticalYield Trial 3 0.0315 60.3% 39.7% Percent Recovery: 0.0135 *100% = 43 % 0.0314 * All calculations were based on Trial 1 Discussion: The most significant source of error in this experiment was human error. The transfer of product to filtration was a source of error. Not all of the solution was successfully transferred over. Also, when massing the original unknown, it created a problem because the balance beam fluctuated the mass and there was no “concluded” mass. The function of the nitric acid was to “pull out” the iron content in the sample. The term gravimetric analysis is defined as the set of methods in analytical chemistry, for the quantitative determination of an analyte based on the mass of the solid. The modifications that I would improve for this experiment was to have a fitting lid for each crucible. The reason being is because there were two lids that broke during this experiment. When the lids broke, it decreased in its original mass and affected the overall mass. Another modification that I would change would be to minimize the amount of transfer for the sample. Conclusion: Overall, the result of this experiment was not successful. The reason being is because the percent recovery was significantly low. This may have been due to the lids of the crucibles to break and this may have caused a change in the mass of the precipitate mass.