10.1.3. Microcanonical Ensemble
advertisement

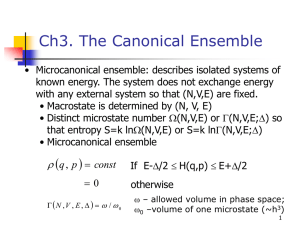
10.1.3. Microcanonical Ensemble Consider a system that is completely isolated from its environment. Since there are no particle and energy exchanges, it is closed and isoenergetic. The corresponding Gibbs ensemble is called the microcanonical ensemble. Its probability density micro is non-vanishing only on the (6N1)-D constant energy surface, where H X E . According to the so-called principle of equal priori probability, the system has an equal chance to be in each of its accessible instantaneous states. Hence, we have 1 (10.6) micro X , E H X E E where the normalization constant (E) is given by E d 6N X H X E (10.7) As discussed in §10.1.1, the next step is to assume that the microcanonical ensemble averages are equal to the corresponding long-time averages, i.e., T 1 dt f X t , t T T 0 6N d X micro X f X lim (10.8) where f X is any smooth function representing some measurable physical quantity. A system satisfying (10.8) is called ergodic. Obviously, (10.8) is meaningful only if the right-handed side is independent of X, or, equivalently, almost all of its initial values X t 0 . The qualifier “almost all”, which stands for “all except for the points in a set of zero measure”, was inserted for mathematical rigor. The implication of (10.8) is as follows. Let us divide the constant energy surface into an arbitrarily large number of small cells. If the system is ergodic, then we expect the system point X(t) to pass through every cell eventually. Furthermore, the fraction of time it spends in a cell is proportional to the weight of that cell in the ensemble average. The qualifier “almost all” denotes the fact that these observations may not remain valid if we replace “cells” with “points”, i.e., there may be points on the energy surface which the system point can get arbitrarily close to, but never actually reach. Further justification of the microcanonical ensemble is offered by the ergodic theorem, which states that the microcanonical ensemble is the only time-independent probability density on the energy surface for an ergodic system. The converse, that a system for which the only time-independent probability density is the microcanonical ensemble is ergodic, is also true. Unfortunately, actual application of this theorem is hindered by the extreme difficulties involved in the proof that any system of real physical interest is actually ergodic. An often cited proof was given by Sinai for the simple case of a gas of hard spheres. One then argues that, if such a simple system can be ergodic, so should any real physical system that is much more complicated. Now, ergodicity only ensures the equivalence between the ensemble and long-time averages for systems in thermodynamic equilibrium. It does not address the problem concerning the actual approach to the equilibrium state, starting from any arbitrary initial state. According to the Liouville’s theorem, the probability density in the neighborhood of any member of the ensemble is a constant independent of time. Hence, any initial inhomogeneities in (X) cannot be smoothed out by the time evolution of the members of the ensemble. The equilibrium state, whose probability density is by definition homogeneous, is therefore seemingly unattainable. One way to extract ourselves from this possible contradiction with the most fundamental principle of thermodynamics is to introduce the concept of probability density mixing. Thus, although the total volume of the incompressible probability fluid remains unchanged, its shape can evolve into the meandering form as depicted by Fig.10.1. This type of behavior is called mixing since it recalls the mixing of 2 immiscible liquids such as water and oil. Operationally, equilibrium is reached when the inhomogeneities in (X) can no longer be resolved by available experimental measurement. In a mathematical description, one can divide the constant energy surface into small cells of linear sizes equal to the desired resolution, and define a coarse-grained probability density by averaging (X) over each cell. A rigorous treatment of mixing is too technical to be described here. In suffices to mention a theorem that says all mixing systems are ergodic but the converse is not necessarily true. In particular, the hard sphere gas was shown by Sinai to be mixing.