Ensemble equivalence in the thermodynamic limit
advertisement

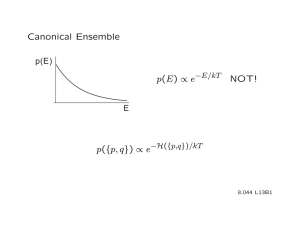
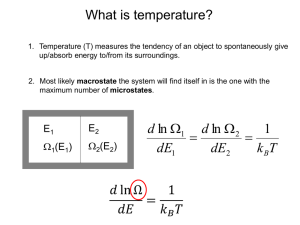
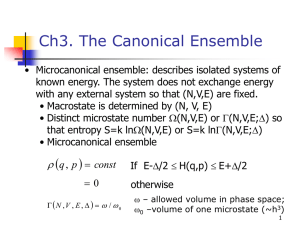
Ensemble equivalence The problem of equivalence between canonical and microcanonical ensemble: canonical ensemble contains systems of all energies. How come this leads to the same thermodynamics the microcanonical ensemble generates with fixed E ? Heuristic consideration 0.08 N=20 kBT=1 P ( E ) dE P(E) 0.06 3 0.04 P(E ) 2 0.02 0.00 = Probability of finding a system (copy) in the canonical ensemble with energy in [E,E+dE] 0 20 3 E 2 3 2 40 60 3 E 80 N k BT 1 N 3 E E example for monatomic ideal gas N 1 2 0 example here with N=20, kBT=1 100 U E 3 2 N k BT N 1 E2 dE e N k BT 2 30 5.5 e E N k BT 3 20 1 30 2 example here with N=20, kBT=1 In the thermodynamic limit N overwhelming majority of systems in the canonical ensemble has energy U= <E> Next we show: E Var [ E ] E and E 1 N E 2 2 E is a general, model independent result Brief excursion into the theory of fluctuations V ar [ X ] X X Measure of: average deviation of the random variable X from its average values <X> 2 From the definition of <f(x)> as: f (X ) We obtain: X X 2 f ( X ) X X 2 X 2 X 2 X X X 2 2 2 X 2 X 2 X X X 1 X 2 X 2 Energy fluctuations Goal: find a general expression for E 0 E E 2 2 2 E 2 E U 2 We start from: U E of the canonical ensemble E U C V T T V E e e T E T k BT T 2 E e 2 1 2 e E E e E 2 E E E E E e e E e E e 1 k BT E E 1 2 k BT 1 k BT 2 E e E e E 2 E 2 E 2 E e E e E 2 CV T E 2 2 E E 2 E E 2 2 T CV U 2 U and CV are extensive quantities and E U N E 2 E E 2 CV N 2 1 N and E E E 2 E E 2 1 N As N almost all systems in the canonical ensemble have the energy E=<E>=U Having that said there are exceptions and ensemble equivalence can be violated as a result An eye-opening numerical example Let’s consider a monatomic ideal gas for simplicity in the classical limit We ask: What is the uncertainty of the internal energy U, or how much does U fluctuate? For a system in equilibrium in contact with a heat reservoir U fluctuates around <E> according to U E E With the general result E E E For the monatomic ideal gas with U E E 3 2 N k BT T kB 3 2 E 2 2 T CV U E E 3 2 NkB N k BT and N k BT 1 2 3 For a macroscopic system with N N A 6 10 23 2 T k B CV E T U CV 3 2 k B CV NkB 2/3 3 N k B T N 2 10 12 Energy fluctuations are completely insignificant 0.82 1 N Equivalence of the grand canonical ensemble with fixed particle ensembles We follow the same logical path by showing: particle number fluctuations in equilibrium become insignificant in the thermodynamic limit N N 2 N 2 remember fugacity z e 2 N We start from: N N (N ) N z Z (N ) N 0 z ln Z G z ln N z Z (N ) N 0 ln Z G ln N N z Z (N ) N 0 N 0 With z Z (N ) N N 0 we see N z 1 Z (N ) N 0 N 0 z ln Z N G z z z N 1 N z Z (N ) z N N z Z (N ) N 0 N 0 N z Z (N ) 1 z N 2 N 1 N N 1 N N z Z (N ) z Z (N ) Nz Z (N ) N z Z (N ) N 0 N 0 N 0 N 0 z N N z Z (N ) N 0 N z Z (N ) N 0 N z Z (N ) N 0 N 2 N 2 z 2 N 2 ze N 2 z 1 N 2 z 1 N z z L n Z G z z Remember: k B T ln Z G P (T , ) V With z z 1 ln Z G P (T , )V k BT z P (T , )V z L n Z z z G z z z z k BT 2 1 1 P (T , )V P k BT V 2 k BT , P V T , T ,V With N N V 1 v 2 V 2 V P 2 P 1 2 2 v 1 v v 2 With P P v and again v P 2 2 P 1 v 3 1 v v 1 1 v P v 1 P v Using the definition of the isothermal compressibility T P 2 N 2 2 N N 2 k BT V N N 2 2 2 k BT T v N k BT V v 0 N 2 T k BT N T / v Particle fluctuations are completely insignificant in the thermodynamic limit 1 v v P T