Ketones A-B
advertisement

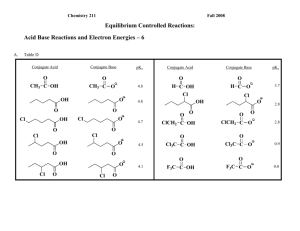
Chemistry 211 Fall 2008 Equilibrium Controlled Reactions: Acid Base Reactions & Energy Relationships - 4 Table B Conjugate Acid CH2: - CH3 O - ~27 O: O - 16 10 O H CH3 C O: - : : 20 : : - H - 15.7 O CH3 C CH3 C CH2 : CH3 :OH ~35 O O - NH : : NH H2O: : : : : : NH2 - NH2 : : : : CH2 : CH3 CH3 C : : ~36 CH3 C 16 O : NH2 - O : : : : : NH3 CH3 C NH - pKa CH3 O : CH3 OH ~40 ~37 CH3 CH3 NH2 Conjugate Base ~42 CH3: - CH4 Conjugate Acid : : CH3 pKa : : CH3 Conjugate Base 4.8 A. Based upon the data in Table B: 1. List the following compounds in the order of decreasing acidity and explain how you determined your order of acidities from the data in Table B. CH3 : : : NH2 OH Using the pKa’s in table B, we were able to isolate the variable in each molecule and compare pKa’s for the NH2, the CH3, and the OH rather than that of the entire structure. The higher the pKa, the stronger the acid. Acid-Base Rxns & Energy Relationships-4 2 2. Which of the following ions has the lowest energy? Which has the highest energy? Explain the reasoning behind your choices with references to data in Table B. O C CH2 : NH : : C O: : : C O O The first ion is similar to 4.8 therefore it has the lowest pKa, making it the lowest because pKa and energy are directly proportional. The second ion is most closely related to 16 and the third ion is closest in structure to pKa 20, therefore because pKa is directly related to energy, energy increases as pKa increases (third ion has the greatest energy.) B. Suggest a theoretical explanation for the effects observed above in terms of electron nuclear attraction and/or electron-electron repulsion. Carbon has the lowest number of protons, therefore has less attraction to the electrons. Oxygen has the most protons and has the most attraction to electrons. C. Using the theory developed in Part B, select the most acidic proton(s) in the following compound. Then explain the process and logic that you used to make your choice. H H H H N H C H C C H C C H H O C H H H H Acidity is indirectly related to pKa of the conjugate base, which is directly related to Gibbs Free Energy. Therefore, the protons with the most energy, have the higher pKa, and lower acidity. 3 Acid-Base Rxns & Energy Relationships-4 Out of Class Applications for Acid Base Reactions &Energy Relationships - 3 A. Reading Assignment: in CGWW: pp. 181-191. B. Activities: For each of the following compounds, use the theory developed in this activity to select the most acidic proton(s): H H H H H H H N C C O H H H H C N C O C H H C C H H C H H H H H Then explain the process and logic that you used to reach your conclusions. 2. Which of the following ions should have the lower energy. : : : 1. : : : - - P H CH2 : : : : S: CH3 CH3 CH2 CH2 Explain your choice on the basis of the theory developed in this activity. CH2 Acid-Base Rxns & Energy Relationships-4 3. 4 For each of the following acid-base reactions, use the theory developed in this activity to predict whether the equilibrium constant should be > or < 1. Explain the logic that led you to your conclusion. a. O : : H + b. + NH2 CH2 : O: - H : + : H : + : CH3 O: - : NH : : : : O H 5 Acid-Base Rxns & Energy Relationships-4 4. For the following pair of equilibrium controlled reactions, predict which one should produce more product at equilibrium. Explain your choice on the basis of the theory developed in this activity. -S O + + C. O S -S NH - - HN S Appropriate Problems in CGWW: CH 8: p. 207, Problem 4, first 2 molecules. E. Nomenclature of Haloalkanes (alkyl halides) 1. Reading assignment: CGWW: Ch. 2 p. 33 2. Tutorials: References: a. http://chemistry.boisestate.edu/people/richardbanks/organic/nomenclature/organicnomenclature1.htm Developed by Richard C. Banks, Professor of Chemistry, Boise State University Provides questions with answers Section Organic Halides b. http://www.cem.msu.edu/~reusch/VirtualText/nomen1.htm Developed at Michigan State University Provides naming rules and examples Sections: no coverage of alkyl halides Acid-Base Rxns & Energy Relationships-4 c. http://www.acdlabs.com/iupac/nomenclature Developed by Advanced Chemistry Development Laboratories 6 (Gives detailed IUPAC rules for nomenclature.) Recommendations 1993 R-5 Applications to Specific Classes of Compounds R-5.3 Halogen, Nitro, Nitroso, Azo, Diazo and Azido Compounds R-5.3.1 Halogen compounds. 3. Applications a. Name the following: F Br Cl I Cl Cl b. Draw structural formulas for the following compounds: 2-ethyl-1,1-difluorocyclobutane 2,5-dimethylhexane 1-bromo-1-propylcycloheptane I 4-t-butyl-2-chloro-6,6-diiodononane Cl