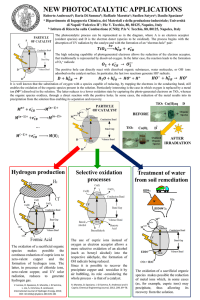
4.Salt-Formation_by_Redox_Reactions_Sellbstlern
advertisement

Formation of Salts by REDOX-Reaction (Introduction) Definition Reactions by which electrons are transferred Origin of the term Redox = reduction and oxidation The two always take place together. Oxidation: classical meaning: Reaction of an element with oxygen E.g. Oxidation of C to CO2, H2 to H2O, iron to iron-oxide (rust)...... However, the concept oxidation is used more widely: e.g., With the formation of NaCl from sodium and chlorine one says that sodium is oxidized, meaning that electrons are taken away from sodium, or in other words: sodium donates electrons. Chlorine is reduced, meaning that electrons are given to chlorine, or in other words: chlorine accepts electrons. In some books one finds the following definitions: - Oxidation means electron release/donation - Reduction means electron acceptance Non-metals generally oxidize metals because non-metals have the higher electronegativity (EN) Formation of Salts by REDOX-Reactions (Electron transfer reactions) At the example of the synthesis of cooking salt Step by step derivation of the reaction equation: Half reactions Na -------------> Na+ + e- x2 Oxidation: (Electron release) Sodium-metal is oxidized to Na+ions. Cl2 + 2 e-----> 2 Cl- Reduction: (Electron acceptance) Cl2 gas is reduced to Cl- ions. ------------------------------------------------------ --------------------------------------------2 Na + Cl2 (+ 2 e-) ----> 2 Na + + 2 Cl-(+ 2 e-) Ion equation 2 Na + Cl2 -----> 2 NaCl Material equation Definitions Reducing-agents reduce other materials. I.e.(this means) they are electron donators and are oxidised themselves. Oxidising-agents....... Which is the reducing agent and which is the oxidizing agent with the reaction above? Experiment: Combustion of magnesium Observation: ENERGY It burns with a bright flame - brighter and hotter than the reaction of sodium + chlorine Conclusion: The released lattice energy of the reaction of magnesium + oxygen is bigger than the one of Na + Cl2. Explanation: Compare both ions. Ion charge: Mg2 +> Na+ O2-> ClIon size: Mg2 + <Na+ O2-< ClBoth criteria indicate that MgO should have the larger lattice energy. PRODUCT a white powder, dissolves in water - 2 material classes are possibly; conducts electricity in solution => salt-like material Which ions are produced? Mg ----> Mg2+ + 2 e- x2 O2 + 4 e- ----> 2 O2- ---------------------------------2 Mg + O2 ----> 2 Mg2+ + 2 O2- Half reactions Oxidation: e- donation Mg metal is oxidized to Mg2 + ions. Reduction: e- acceptance O2 gas is reduced to O2 ions. Ion equation 2 Mg + O2 ----> 2 MgO Material equation Mg acts as a reducing agent, O2 as an oxidizing agent. Experiment: Aluminium and Bromine Observations (focus on energy) Product: a white smoke, therefore a white powder The released lattice energy of this reaction is between the ones of Na + Cl and Mg + O2, because Br- is large but only weakly charged, whereas Al3+ is small and highly/strongly charged. Half reactions Al ----> Al3+ + 3 eBr2 + 2 e- ----> 2 Br- x2 x3 Oxidation: e- donation Al-metal is oxidized to Al3 + ions Reduction: e- acceptance Br2 is reduced to Br- ions. -------------------------------2 Al + 3 Br2 ----> 2 Al3+ + 2 Br- Ion equation 2 Al + 3 Br2 -----> 2 AlBr3 Material equation Remark: Non-metals form 2-atomic ions, except phosphorus forms P4- and sulphur forms S8-molecules. Different Reaction Rates of the three states of aggregation Liquid bromine is more reactive than the gaseous chlorine or oxygen because its smallest particles are much denser than with gases. At the same time liquids more reactive than solids because their smallest particles are able to move freely in contrast to solids, where the smallest particles are confined and only vibrate at their lattice positions.