Supplementary Figure Legends - Word file (25 KB )
advertisement

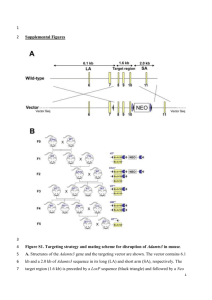
Supplementary Fig. 1 Differential expression pattern of COUP-TFII using knockin lacZ reporter as a marker in the vasculature. (a-d). Using COUP-TFII/lacZ knock-in mice, we examined the expression pattern of COUP-TFII in the vascular tissue at E10.5 (a and b) and E11.5 (c and d). COUP-TFII is expressed in the endothelial cells of the anterior cardinal vein (ACV) (a, arrows), umbilical vein (UV) (c, arrows) and vitelline vein (VV) (d, arrows), but not in the endothelial cells of dorsal aorta (DA) (a, arrows), internal carotid artery (ICA) (b, arrows), and umbilical artery (UA) (c, arrows). However, COUP-TFII is highly expressed in the smooth muscle cell layer surrounding the DA (a), ICA (b) and UA (c). Fig. 2 Model of cell autonomous versus non-cell autonomous functions. To generate wild type and COUP-TFII null ES cells containing the ROSA26 allele, COUP-TFII+/- mice were first crossed with the ROSA26 line1 to generate COUPTFII+/-; ROSA26/+ compound heterozygote. The compound heterozygote was then crossed to COUP-TFII+/- mice to obtain blastocysts from dated pregnant females. The stable ES cell lines with COUP-TFII+/+; ROSA26/+ and COUP-TFII-/-; ROSA26/+ genotypes were generated from the isolated blastocysts. To analyze the cellautonomous verse non cell-autonomous function of COUP-TFII, the ES cells were then microinjected into the wild type blastocyst (shown in pink) to generate chimeric embryos2, 3. The cells derived from the ES cells can be identified by X-Gal staining (shown in blue). The mutant cells will not be able to contribute to the tissues where they are required cell-autonomously. In contrast, if mutant cells play a non cell- autonomous function in the tissues, they will be able to contribute as well as the wild type cells. Fig. 3 Endothelial-specific knockout of COUP-TFII by Tie2-Cre. (a) Generation of floxed COUP-TFII and COUP-TFII/LacZ knock-in mice. Using homologous recombination strategy, the targeting vector was inserted into genomic COUP-TFII locus. Upon Cre- mediated recombination, ES cells with floxed COUPTFII and LacZ knock-in alleles were generated and used to create mice. Floxed COUP-TFII mice can be used for tissue specific knockout using tissue specific Cre lines. Upon Cre-mediated recombination between these two loxP sites, the expression of lacZ, containing the nuclear localization signal, will be turned on under the control of COUP-TFII promoter, permitting the determination of the recombination event. In addition, the LacZ knock-in mice will facilitate the monitoring of the cell specific expression pattern of COUP-TFII during development. (b-d) Whole embryo X-Gal staining showed the Cre-mediated recombination by crossing Tie2-Cre with either R26R reporter mice or floxed COUP-TFII mice at E9.5. X-Gal staining was apparent in all endothelium of reporter mice subsequent to Tie2Cre crossing with R26R reporter mice (c), but not the control littermate (b). Only the venous endothelium was marked by X-Gal staining in case of Tie2-Cre/+; COUPTFIIflox/+ embryos (d). It is notable that the staining present in dorsal aorta and brachial arch arteries of the Tie2-Cre/+; R26R/+ reporter embryo (c) was missing in Tie2-Cre/+; COUP-TFIIflox/+ embryos as indicated by arrows (d). (e-h) Cre- mediated recombination in Tie2-Cre/+; R26R/+ and Tie2-Cre/+; COUPTFIIflox/+ embryos at E9.5. At cardinal vein level, the endothelial-specific excision visualized by X-Gal staining was observed in all endothelial cells in Tie2-Cre/+; R26R/+ reporter embryos (e), while Cre-mediated recombination was only seen in venous endothelium in Tie2-Cre/+; COUP-TFIIflox/+ embryos (f). Similarly, X-Gal positively stained cells were seen at both arterial and venous endothelium in Tie2Cre/+; R26R/+ reporter embryos (g), whereas the X-Gal positive signals were absent from arterial endothelium in Tie2-Cre/+; COUP-TFIIflox/+ embryos (h) at umbilical arteries (UA) and veins (UV) region. Since the inserted LacZ gene containing a nuclear localization signal in floxed COUP-TFII allele, the appearance of the nuclear X-Gal staining pattern is different from cytoplasmic staining pattern of R26R reporter line. A, aorta; V, vein. Fig. 4 The endothelial-specific COUP-TFII null mutants exhibited a variety of vascular defects. (a and b) The endothelial-specific COUP-TFII null mutants die in utero by E12. Mutant embryos at E11.5 show widespread hemorrhage (b) compared with the littermate (a). (c and d) The major histopathological features of mutants who survived at E11.5 show thin and well-dilated vessels. Transverse sections of the E11.5 embryos were stained by hematoxylin and eosin. The dilated vessels at the posterior cardinal vessel level were observed in the mutant embryo (d) but not in the control embryo (c). Also noted is the cell layers that separated the artery and vein (indicated by arrows) are greatly reduced in the mutant (d) as compared to the littermate control (c). A, aorta; V, vein. Fig. 5 The hematopoietic-specific markers in hematopoietic cell clusters of the mutant. The identities of these hematopoietic cell clusters from mutant veins were further confirmed using anti-CD45 (a, b) and anti-c-kit (CD117) (c) antibodies in immunostaining. CD45 antigen is a tyrosine phosphatase, which is presented on the hematopoietic cells. Cells arised from the head vein (a) and umbilical vein (b) of the mutant are round in appearance and express CD45 (brown), indicated that they are hematopoietic cells. As c-Kit had been shown to express on both hematopoietic stem cells (HSC) and endothelial cells (EC)4. The positive signal (brown) was observed in these two compartments in the mutant umbilical vein (c). Supplemental References 1. Friedrich, G. & Soriano, P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 15131523 (1991). 2. Bradley, A., Evans, M., Kaufman, M. H. & Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309, 255-256 (1984). 3. Nagy, A. et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development 110, 815-821 (1990). 4. Bernex, F. et al. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development 122, 3023-3033 (1996).