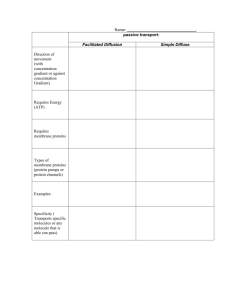
The Endoplasmic Reticulum (ER): Synthesis of Integral
advertisement

CHAPTER 8 (12 NA EDIÇÃO EUROPEIA) CYTOPLASMIC MEMBRANE SYSTEMS: STRUCTURE, FUNCTION, AND MEMBRANE TRAFFICKING OBJECTIVES Emphasize the dynamic nature of the endomembrane system within the cell. Discriminate between regulated and constitutive secretion. Outline research techniques that have elucidated the structure and function of the endomembrane system. Clarify the history behind the discovery and description of endomembrane system organelles. Elucidate the structure and function of the rough and smooth endoplasmic reticulum. Point out the differences between the syntheses of secretory/integral membrane and domestic proteins. Outline the events in the synthesis and transport of membranes through the cell to the membrane. Elucidate the role and sites of glycosylation in the processing of secretory/integral membrane proteins. Elucidate the structure, function and polarization of the Golgi complex. Describe the role of the various types of coated- and non-coated-vesicles in membrane trafficking. Explain the signals used to target proteins to their appropriate cellular location. Describe the steps involved in the process of exocytosis and its triggers. Describe lysosomal structure and function and the diseases caused by lysosome malfunction. Distinguish between phagocytosis, bulk phase endocytosis and receptor-mediated endocytosis. Explain the role of receptors, coated pits, and clathrin-, COPI- and COPII-coated vesicles in the internalization of extracellular materials. LECTURE OUTLINE An Overview of the Endomembrane System and Its Dynamic Nature I. Before the 20th century - stained tissue sections hinted at an extensive membrane network in cytoplasm A. 1940s - EM revealed diverse array of membranous structures in cytoplasm of most eukaryotes 1. Membrane-bound vesicles of varying diameter; containing material of different electron density 2. Long channels bounded by membranes that radiate through cytoplasm; form an interconnected network of canals 3. Stacks of flattened, membrane-bound sacs (cisternae) B. These studies & subsequent biochemical studies showed that eukaryotic cell cytoplasm was subdivided into a variety of distinct membrane-bound compartments 1. Saw distinct organelles in diverse cells from yeast to higher plants and animals 2. The organelles may appear as stable structures, but, in fact, they are dynamic compartments that are in continual flux 3. These organelles have distinct structures & functions but together form an endomembrane system; the individual components function as part of coordinated unit C. Mitochondria & chloroplasts are not part of this interconnected system D. Current evidence suggests that peroxisomes have a dual origin 1. The basic elements of the boundary membrane probably arise from the endoplasmic reticulum, 2. But most of the membrane proteins & soluble internal proteins are taken up from the cytoplasm 321 II. These organelles are part of dynamic, integrated network; materials are shuttled between parts of cell A. Transport vesicles shuttle things between organelles; form by budding from donor compartment 1. Vesicle implies a spherical-shaped carrier; cargo may also be transported in irregular or tubular shaped membrane-bound carriers 2. But the term vesicle is often used, keeping in mind that they are not always spherical B. Transport vesicles move in directed manner, often pulled by motor proteins operating on tracks formed by microtubules & microfilaments of the cytoskeleton C. When they reach their destination, they fuse with acceptor compartment, which receives vesicles' soluble cargo & membrane wrapper D. Exhibit repeated cycles of budding & fusion that move a diverse array of materials along numerous pathways traversing the cell III. Several distinct pathways through cytoplasm have been identified; they fall into two groups: a biosynthetic (secretory) pathway & an endocytic pathway IV. Biosynthetic (secretory) pathway – synthesis in ER (protein) or Golgi (lipid, carbohydrate); altered as pass through Golgi, sent from there to various locations (membrane, lysosome, large plant cell vacuole, etc. A. Many materials made in ER (proteins) & Golgi (complex polysaccharides) fated for secretion from cell B. Two types of secretory activity - constitutive & regulated 1. Constitutive - synthesis & secretion into extracellular space occurs in continual, unregulated manner; most cells do it to form extracellular matrix & plasma membrane itself 2. Regulated - secretory materials are often stored in large, densely packed, membrane-bound secretory granules in cell periphery; secreted after correct stimulus a. Endocrine cells release hormones b. Pancreatic acinar cells release digestive enzymes c. Nerve cells release neurotransmitters C. Proteins, lipids & complex polysaccharides are transported through cell along biosynthetic or secretory pathway; discussion will center on several distinct classes of proteins 1. Soluble proteins discharged from cell 2. Integral proteins of various membranes 3. Soluble proteins that reside within various compartments enclosed by endomembranes (like lysosomal enzymes) V. Endocytic pathway - moves materials or membrane surface into cell from outside to cytoplasmic compartments (endosomes, lysosomes); movement direction is opposite to that of secretory pathway VI. Proteins targeted to specific destinations through sorting signals located on them & receptors in transport vesicle walls that recognize them (analogous to trucks carrying different cargo to various sites) A. Both transport pathways require defined traffic patterns; ensure accurate delivery to correct sites 1. Ex. - salivary gland cell protein trafficking; salivary mucus proteins (made in ER) specifically targeted to secretory granules; lysosome enzymes (also made in ER) specifically sent to lysosome 2. Different organelles also contain different integral membrane proteins; they must also be targeted to particular organelle (lysosome, Golgi cisterna) B. Targeting involves integral membrane proteins, secretory proteins, lysosomal proteins; they are routed to their appropriate cellular destination by virtue of specific addresses (sorting signals) 1. Sorting signals are encoded in protein amino acid sequence or in attached oligosaccharides C. Sorting is facilitated by specific membrane receptors for sorting signals found in particular membranes of endomembrane system or by coats that form on outer surfaces of transport vesicles 1. Specific receptors are on surfaces of budding vesicles 2. Ensures that protein is transported to the appropriate destination 322 3. For most part, machinery responsible for driving this complex distribution system consists of soluble proteins that are recruited to specific membrane surfaces 4. At those surfaces, they perform their designated activities D. Great advances in experimental approaches have been made over last 2 or 3 decades in: 1. Mapping the traffic patterns that exist in eukaryotic cells 2. Identifying the specific addresses & receptors that govern the flow of traffic 3. Dissecting the machinery that ensures that materials are delivered to appropriate cellular sites A Few Approaches to the Study of Cytomembranes I. EM micrographs give detailed view of cell cytoplasm, but little insight into functions of the structures A. Cells perform dynamic processes, but EM portrays only static scenes B. Determining functions of cell organelles required new techniques & innovative experiments C. Such innovations resulted in 1974 Nobel Prize for 3 cell biologists: Christian De Duve (University of Louvain in Belgium), Albert Claude & George Palade (both of Rockefeller University) II. Insights gained from autoradiography - can detect location of radioactively labeled materials in cell A. Pancreas acinar cells have a particularly extensive endomembrane system; ideal for study by autoradiography 1. The cells function primarily in synthesis & secretion of digestive enzymes 2. Enzymes are shipped via ducts from pancreas, where they are synthesized, to small intestine to degrade ingested food matter B. James Jamieson & George Palade - worked with pancreas acinar cells; followed secretory protein from synthesis to secretion & determined individual steps even though all of them occurred simultaneously 1. Able to observe steps of single cycle of secretion from start to finish 2. Autoradiography allows visualization of biochemical processes by allowing investigator to determine the location of radioactively labeled materials within cell C. Procedure - section tissues containing radiolabel & locate hot digestive enzymes with autoradiography 1. Incubate tissue slices with hot (radioactive) amino acids briefly —> incorporated into digestive enzymes as they are made on ribosomes 2. Fix tissues; tissue sections containing radioactive isotopes were then covered with thin photographic emulsion layer, which is thus exposed to radiolabel emanating from radioisotopes within tissue 3. Sites in cell with radiolabel are highlighted with developed silver grains in overlying emulsion 4. If label, wash & harvest immediately, label appears first over RER —> RER was site of synthesis III. Insights from pulse-chase trials (Palade & Jamieson) - show secretory protein path after synthesis to their site of discharge A. Expose to hot amino acids briefly (pulse) followed by a wash to remove excess isotope from tissue 1. Pulse refers to the brief incubation with radioactivity during which labeled amino acids are incorporated into protein B. Transferred tissue to medium with unlabeled amino acids (chase), which lasts for varying time periods 1. During this period, protein synthesis continues using nonradioactive amino acids 2. The longer the chase, the farther the hot (radioactive) proteins made during the pulse will have traveled from their synthesis site (the RER) within the cell C. One can see wave of radioactivity moving through cell, discern pathway sequence - RER was synthesis site & see rest of pathway from one location to the next until the process is complete 1. Defined the secretory (biosynthetic) pathway & tied a number of seemingly separate membranous compartments into an integrated functional unit IV. Insights gained from use of green fluorescent protein (GFP) – scientists can follow within their own eyes the dynamic movements of specific proteins as they move within single living cell; do not have to kill cells 323 A. GFP is small protein from certain jellyfish that emits a green fluorescent light 1. Its gene has been isolated & can be fused to DNA encoding protein to be studied 2. The resulting chimeric (composite) DNA is introduced into cells that can be observed in scope 3. Once inside cell, chimeric DNA expresses chimeric protein consisting of GFP fused to end of protein to be studied 4. Usually, GFP stuck to end of a protein has little or no effect on its movement or function & protein under study has no effect on fluorescence of attached GFP B. Example: infect cells with vesicular stomatitis virus (VSV) strain in which a viral gene (VSVG) is fused to GFP gene; viruses useful since they turn cells into factory for producing viral proteins 1. These viral proteins are carried like any other protein cargo through the biosynthetic pathway 2. Cell begins to make massive amounts of VSVG protein in RER 3. VSVG then goes to Golgi complex & eventually to the plasma membrane of the infected cell where they are incorporated into viral envelopes 4. Can see relatively synchronous wave of protein movement (green fluorescence) soon after infection 5. Synchrony is enhanced by use of virus with mutant VSVG protein that cannot leave ER of infected cells grown at elevated temperature (40°C) 6. When temperature is lowered to 32°C, the fluorescent GFP-VSVG protein that had accumulated in ER moves synchronously to Golgi complex for various processing events & then to membrane 7. Mutants of this type that function normally at reduced (permissive) temperature, but not at elevated (restrictive) temperatures are described as temperature-sensitive mutants V. Insights gained from the biochemical analysis of subcellular fractions - cell homogenization & organelle isolation techniques were pioneered by Albert Claude & Christian De Duve (1950s & 1960s) A. Homogenize cells; form cytoplasmic membrane fragments, the ends of which fuse to form spherical vesicles (<100 nm dia) B. Vesicles formed from different organelles (nucleus, mitochondrion, plasma membrane, ER, etc.) have varied properties, which allow their separation (cell fractionation) from one another 1. Endomembrane system (primarily ER, Golgi) vesicles form heterogeneous, similar-sized vesicles (microsomes); rapidly (& crudely) purified, then separated further; often retain biological activity 2. Fractionate microsomes into smooth & rough membrane fractions by gradient techniques (Ch. 18) 3. Once isolated, one can determine the biochemical composition of various fractions C. Example of uses & findings - vesicles from different parts of Golgi were found to have enzymes that add different sugars to the ends of growing CHO chains of glycoprotein or glycolipids 1. Purify these enzymes from the microsomal fraction; use them as antigens to make antibodies & attach gold particles to the antibodies, locations of which in Golgi membranes can be seen in EM 2. Revealed role of Golgi complex in stepwise assembly of complex carbohydrates D. Example: identification of proteins in cell fractions taken to new level using sophisticated proteomic technology; isolate organelle, extract & separate proteins & then identify them by mass spectrometry 1. Hundreds of proteins can be identified simultaneously, providing a comprehensive molecular portrait of any organelle that can be prepared in a relatively pure state 2. For example, a simple phagosome, containing an ingested latex bead had >160 different proteins, many of which had never before been identified or were not known to be involved in phagocytosis 3. Several proteins were included that were characteristic of ER, leading to new appreciation of the ER's role in phagocytosis VI. Insights gained from use of cell-free systems – isolated parts of cell studied for their capabilities A. These cell-free systems (which do not contain whole cells) provide information about complex processes that were impossible to study using intact cells B. George Palade, Philip Siekevitz, et al. (Rockefeller University, 1960s) – studied properties of rough microsomal fraction 324 1. Stripped rough microsomal preparation of its attached particles & found that isolated particles (ribosomes) could synthesize proteins when provided with the required cytosol ingredients 2. Newly synthesized proteins were released into the aqueous fluid in test tube 3. When same experiments were conducted with complete rough microsomal fraction, the proteins were not released into incubation medium but were trapped within membranous vesicle lumens 4. So microsomal membrane was not needed for protein synthesis, but for sequestering newly made secretory proteins within ER cisternal space C. Over the past few decades, cell-free systems have been used to identify the roles of many of the proteins involved in membrane trafficking; example below of budding from liposomes 1. Cell-free liposomes (vesicles whose walls consist of an artificial bilayer created from purified phospholipids) used to study specific roles of proteins involved in budding 2. Incubate liposomes with purified proteins that normally comprise coats of cell transport vesicles 3. Without added coat proteins —> no vesicle budding; with it —> get budding 4. Such reconstitution of cellular processes in vitro has been useful in this & other studies 4. They could determine the proteins that bind to the membrane to initiate vesicle formation, those proteins responsible for cargo selection & those that sever the vesicle from the donor membrane VII. Insights gained from study of mutants – a mutant is an organism (or cultured cell) whose chromosomes contain one or more genes that encode abnormal proteins A. Mutant gene products vary from the normal; they can cause a characteristic deficiency in the cell carrying the mutation, which is analyzed 1. Determining the precise nature of deficiency gives information on function of the normal protein B. Randy Schekman, et al., Univ. of Ca. – Berkeley – studied genetic basis of secretion using yeast cells 1. Why he used yeast cells - few genes, small, single-celled & able to be cultured easily, can be grown as haploid so mutants seen; haploid for majority of life cycle; allows easier deficiency detection 2. Gene mutation in haploid yields observable effect; can’t mask presence of abnormal gene with normal one 3. Yeast ER simple & directly connected to outer membrane of nuclear envelope; vesicles bud from ER, travel to Golgi cisternae where they fuse 4. Find genes involved in secretory pathway by screening for mutant cells with abnormal distribution of cytoplasmic membranes (SEC genes) 5. Found mutation in gene for protein involved in vesicle formation at ER membrane —> in absence of vesicle formation, cells accumulated expanded ER cisternae 6. Found another mutation in gene encoding a protein involved in vesicle fusion —> if this gene is defective, cells amass an excess number of unfused vesicles 7. Many mutants that disrupt secretory pathway have been found, cloned & sequenced; mutant proteins have been isolated; homologous proteins (with related sequences) found in mammals VIII. Lessons learned from these techniques A. Dynamic activities of endomembrane systems are highly conserved B. Processes similar in all organisms (yeast, plant, insect & human cells); done with remarkably similar proteins (despite their structural diversity, these cells have underlying molecular similarities) 1. Some proteins doing similar things in different (often widely divergent) species are interchangeable 2. Mammalian cell-free systems can often use yeast proteins to facilitate vesicle transport 3. Researchers can "cure" yeast biosynthetic pathway mutants by genetically engineering them to carry normal mammalian genes The Endoplasmic Reticulum (ER): Background Information and General Functions I. History and general description - first detected in 19th century 325 A. Vague cytoplasmic network seen in stained cells (ergastoplasm) 1. In pancreas cells, ergastoplasm seen to disappear upon starvation & reappear when animal fed 2. Concluded ergastoplasm in pancreas makes digestive juices B. Later seen in EM by Porter who renamed it endoplasmic reticulum II. Endoplasmic reticulum (ER) is divided into 2 broad categories - rough & smooth; both enclose space so cytoplasm divided into cytosolic & luminal (cisternal) space; contents of the 2 spaces are quite different A. Fluorescently labeled proteins & lipids can diffuse from one type of ER into the other, indicating that their membranes are continuous 1. The 2 types of ER share many of the same proteins & engage in certain common activities (synthesis of certain lipids & cholesterol) 2. At the same time, numerous proteins are found only in one or the other type of DNA 3. Thus, RER & SER have different structures & functions, which can be traced to the presence of different proteins in the 2 compartments B. Smooth ER (SER) - typically tubular; interconnecting pipeline system; curves through cytoplasm; lacks associated ribosomes; when cells are homogenized, it fragments into smooth-surfaced vesicles C. Rough ER (RER) – extensive organelle with ribosomes attached to RER on cytosolic surface; made mostly of cisternae (interconnected network of flattened sacs); space inside appears continuous 1. RER is continuous with nuclear envelope outer membrane (it has ribosomes on cytosolic surface) 2. When cell is homogenized, RER fragments into rough-surfaced vesicles 3. Because they have different densities, rough & smooth vesicles can be readily separated by density gradient centrifugation & then studied D. Different cell types contain varying amounts of either one ER type or other; depends on cell activities 1. Cells that secrete large amounts of proteins (pancreas or salivary gland cells) —> lots of RER III. Smooth ER functions - extensively developed in many cells (skeletal muscle, kidney tubules, steroidproducing endocrine cells); its specific proteins vary cell-to-cell depending on functions of cell’s SER A. Synthesis of steroid hormones in gonad & adrenal cortex endocrine cells B. Detoxification in liver of many organic compounds (barbiturates & ethanol), whose chronic use can lead to SER proliferation in liver cells; detoxification carried out by oxygen-transferring enzymes 1. These oxygenases, like cytochrome P450s, convert these compounds into more hydrophilic derivatives so that they can be more easily & readily excreted 2. Sometimes the oxygenases create carcinogens; relatively harmless benzo[a]pyrene formed when meat charred on a grill is converted into potent carcinogen by SER detoxifying enzymes 3. Such enzymes have low substrate specificity; oxidize 1000s of different hydrophobic compounds 4. Cytochrome P450s metabolize many prescribed medications; genetic variation in these enzymes among humans may explain differences between people in drug effectiveness & side-effects C. Sequestering Ca2+ ions within cisternal space; their release triggers specific cell activities 1. SER contains a high concentration of Ca2+-binding proteins 2. Regulated release of Ca2+ ions from SER triggers specific cellular responses, like skeletal muscle cell contraction & fusion of secretory vesicles with the plasma membrane III. Rough ER functions - predominantly export or membrane protein synthesis (pancreatic acinar cells, mucussecreting cells of digestive tract lining; early studies done on these cells) A. Organelles of protein-secreting, glandular epithelium cells are distinctly polarized along cell tall axis (from basal to apical end); reflects flow of secretory products from synthesis to discharge 1. Nucleus & extensive RER cisternae found near cell basal surface near blood supply; RER is site of synthesis proteins, carbohydrate chains & phospholipids that move through cytomembrane system 2. Golgi complex is located in central region of cell 3. Apical surface faces duct lumen that will carry secretory product out of organ 326 4. Cell apical end contains membrane-bound secretory vesicles whose contents are released upon arrival of appropriate signal B. It was found that RER is secretory protein synthesis site (starting point of biosynthetic pathway) in pancreatic acinar cells 1. Other examples found later - intestinal goblet cells (secrete mucoproteins), endocrine cells (polypeptide hormones), plasma cells (antibodies), liver cells (blood serum proteins) The Endoplasmic Reticulum (ER): Synthesis of Proteins on Membrane-Bound vs. Free Ribosomes I. Further experiments revealed that polypeptides are synthesized at 2 distinct locales within cell A. Some proteins are made on ribosomes attached to cytosolic surface of RER membranes 1. Proteins secreted from cells 2. Integral membrane proteins 3. Soluble proteins that reside within compartments of endomembrane system (ER, Golgi complex, lysosomes, endosomes, vesicles, plant vacuoles) B. Other polypeptides made on “free” ribosomes (not attached to ER) & then released into cytosol 1. Proteins destined to remain in cytosol (enzymes of glycolysis, cytoskeleton proteins) 2. Peripheral proteins of inner cell membrane surface (spectrins, ankyrins; weakly bonded to membrane's cytoplasmic surface) 3. Proteins that are transported to nucleus 4. Proteins to be incorporated into peroxisomes, chloroplasts, mitochondria; latter 2 groups made in cytosol & imported fully formed (posttranslationally) across membrane into appropriate organelle II. Why are proteins made at different cell sites & how are they identified? - Signal Hypothesis; earned Nobel for Medicine (1999); Günter Blöbel, David Sabatini & Bernhard Dobberstein (Rockefeller U., early 1970s) A. Suggested & demonstrated that the site of protein synthesis is determined by information (amino acid sequence) contained in N-terminal portion of protein (first part to emerge from ribosome) 1. Secretory proteins have N-terminal signal sequence that directs emerging protein & ribosome to ER 2. Signal sequence triggers attachment of protein-making ribosomes to ER & protein movement into cisternal space through protein-lined, aqueous ER channel as it is being made (cotranslationally) B. Some transport into ER is posttranslational - protein is made totally in cytosol & then imported into ER 1. Goes through same channels as in cotranslational pathway; similar to mechanism of mitochondrial & peroxisomal transport 2. Pathway is used much more heavily in yeast than in mammalian cells for import into ER; yeast can survive without cotranslational transport even though they grow more slowly than normal cells C. Signal hypothesis has been substantiated by a large body of experimental evidence 1. Blöbel's concept that proteins contain their own "address codes" has been shown to apply in principle to virtually all types of protein trafficking pathways throughout cell III. Steps in synthesis of secretory/lysosomal/plant vacuolar protein on membrane-bound ribosomes A. mRNA for secretory/lysosomal/plant vacuolar protein binds to free ribosome (same as those used for domestic proteins) from pool; these ribosomes are not attached to a cytoplasmic membrane B. N-terminal aminos emerge from ribosome with signal sequence (6-15 hydrophobic amino residues); targets nascent polypeptide & ribosome for ER 1. The signal sequence targets the nascent polypeptide to the ER membrane (a nascent polypeptide is one in the process of being synthesized & thus is not yet fully assembled) 2. Signal sequence leads to compartmentalization of polypeptide within ER lumen 3. Signal is usually found at or near N-terminus, but occupies an internal position in some polypeptides C. Signal sequence is recognized by signal recognition particle (SRP) as it exits ribosome; SRP in mammalian cells consists of 6 distinct polypeptides & a small RNA molecule (the 7S RNA) 327 D. E. F. G. H. 1. SRP binds to nascent polypeptide's signal sequence & ribosome (Step 1), temporarily arresting further synthesis of polypeptide Bound SRP serves as tag allowing entire complex (SRP-ribosome-nascent polypeptide) to bind to SRP receptor on ER cytosolic surface specifically; this binding occurs through at least 2 distinct interactions 1. First distinct interaction is between SRP & SRP receptor 2. The other interaction is between ribosome & translocon The translocon is a protein-lined channel embedded in the ER membrane through which the nascent polypeptide is able to move in its passage from the cytosol to the ER lumen 1. Prokaryotic translocon 3D structure was determined by X-ray crystallography & revealed presence of a pore within translocon in shape of an hourglass 2. The pore had a ring of 6 hydrophobic amino acids situated at its narrowest diameter 3. In the inactive (nontranslocating) state, which was the state in which the structure was crystallized, the opening in the pore ring is plugged by a short helix 4. This plug is proposed to seal the channel, preventing the unwanted passage of calcium & other ions between the cytosol & the ER lumen Once the SRP-ribosome-nascent chain complex binds to the ER membrane (Step 2), the SRP is released from its ER receptor & the ribosome is attached to translocon's cytosolic end & then…… 1. The nascent polypeptide's signal sequence is inserted into the translocon's narrow aqueous channel (Step 3) 2. It is proposed that contact of signal sequence with the translocon interior leads to displacement of the plug & opening of the passageway Growing polypeptide is then translocated through hydrophobic pore ring & into ER lumen (Step 4) 1. The pore ring seen in crystal structure has a diameter (5-8 Å), considerably smaller than that of a helical polypeptide chain, so it is presumed that pore expands as nascent chain traverses channel 2. Expansion is possible because the residues that make up the ring are situated on different helices Upon translation termination & completed polypeptide's passage through translocon, the membranebound ribosome is released from ER membrane; helical plug is then reinserted into translocon channel IV. GTP is involved in secretory protein synthesis - several steps are regulated by its binding or hydrolysis A. G-proteins (GTP-binding proteins) play key regulatory roles in many different cellular processes 1. G-proteins exist in at least 2 alternate conformations: active GTP-bound & inactive GDP-bound form; the 2 conformations have different abilities to bind other proteins 2. Thus, G-proteins act like molecular switches turning specific processes on and off; the GTP-binding proteintypically turns process on & hydrolysis of bound GTP to GDP turns process off 3. Also GTP-binding-proteins generally require accessory proteins to carry out their function B. SRP & SRP receptor (2 major interactants in the above process) are both G proteins (unusual) 1. Hydrolysis of GTP bound to these two proteins occurs between steps 2 & 3 & triggers the release of the signal sequence by the SRP & its insertion into the translocon The Endoplasmic Reticulum (ER): Processing of Newly Synthesized Proteins in the Endoplasmic Reticulum I. As nascent polypeptide enters RER cisterna, it is acted upon by a variety of enzymes located within either the membrane or lumen of the RER A. Signal peptide on N-terminus of nascent polypeptide is removed from most of the nascent proteins by a proteolytic enzyme, the signal peptidase B. Carbohydrates are added to nascent protein by enzyme oligosaccharyltransferase 1. Both signal peptidase & oligosaccharyltransferase are integral membrane proteins residing in close proximity to translocon 2. Both enzymes act on the nascent proteins as they enter the ER lumen 328 II. The RER is a major protein processing plant A. To meet its obligations, RER lumen is packed with molecular chaperones that recognize & bind to unfolded or misfolded proteins & give them opportunity to attain their correct (native) 3D structure B. The ER lumen also contains a number of protein-processing enzymes, like protein disulfide isomerase (PDI) 1. Proteins enter ER lumen with their cysteine residues in the reduced (—SH) state, but theyleave the compartment with many of these residues joined to one another as oxidized disulfides (—S-S—) 2. The formation (& rearrangement) of disulfide bonds is catalyzed by PDI 3. Disulfide bonds play an important role in maintaining the stability of proteins that are present at the extracellular surface of the plasma membrane or secreted into the extracellular space III. The ER is ideally constructed for its role as a port of entry for the biosynthetic pathway of the cell A. Its membrane provides a large surface area to which many ribosomes can attach (an estimated 13 million/liver cell) B. ER cisternae lumen provides local environment that favors protein folding & assembly C. ER cisternae lumen also provides a compartment in which secretory, lysosomal & plant-cell vacuolar proteins can be segregated from other newly made proteins 1. This segregation of newly made proteins in ER cisternae removes them from cytosol 2. It also allows them to be modified & dispatched toward their ultimate destination, whether outside the cell or within one of the cytoplasm's membranous organelles The Endoplasmic Reticulum (ER): Synthesis of Integral Membrane Proteins on Membrane-Bound Ribosomes I. Integral membrane proteins (other than those of mitochondria & chloroplasts) are also synthesized on membrane-bound ribosomes of ER A. These membrane proteins are translocated into ER membrane as they are synthesized (cotranslationally) using the same machinery used for synthesis of secretory & lysosomal proteins 1. Unlike soluble secretory & lysosomal proteins, however, which pass entirely through ER membrane during translocation, integral proteins contain ≥1 hydrophobic transmembrane segments 2. These hydrophobic transmembrane segments are shunted directly from the translocon channel into the lipid bilayer – how can this take place? B. X-ray crystallographic studies of translocon showed translocon to have a clam-shaped conformation with a groove or seam along one side of the wall where the channel might open & close 1. As protein moves through translocon, it is thought that lateral gate in channel continually opens & closes; allows each nascent polypeptide segment to partition itself according to solubility properties 2. Each segment may stay in the aqueous compartment within translocon channel or move into the surrounding hydrophobic lipid bilayer core 3. The segments of nascent polypeptide that are sufficiently hydrophobic will spontaneously dissolve into lipid bilayer & ultimately become transmembrane integral membrane protein segments C. This idea has received strong support from in vitro study in which translocons were given the chance to translocate custom-designed nascent proteins containing test segments of varying hydrophobicity 1. The more hydrophobic the test segment, the greater the likelihood that it will pass through the wall of the translocon & become integrated as a transmembrane segment of the bilayer II. Single-spanning membrane proteins can have an orientation with their N-terminus facing either the cytosol or the ER lumen (& eventually the extracellular space) A. The most common determinant of membrane protein alignment is the presence of positively-charged amino acid residues flanking the cytosolic end of a transmembrane segment B. During membrane protein synthesis, the inner lining of translocon is thought to orient the nascent polypeptide so that the more positive end faces the cytosol 329 III. In multispanning proteins, sequential transmembrane segments have opposite orientations A. For these proteins, every other transmembrane segment has to be rotated 180° before it can exit the translocon B. Studies performed with purified components in cell-free systems suggest that a translocon, by itself, is capable of properly orienting transmembrane segments C. It appears that translocon is more than a simple passageway through ER membrane; it is a complex "machine" that can recognize various signal sequences & perform complex mechanical activities The Endoplasmic Reticulum (ER): Membrane Biosynthesis in the ER I. Membranes thought to arise only from pre-existing membranes (not de novo [new entities from pools of proteins & lipids]) A. Membranes grow as newly made proteins & lipids are inserted into existing membranes in ER; each compartment has unique membranes 1. Membrane components move from ER to virtually every other cell compartment 2. As membrane moves from compartment to compartment in cell, its proteins & lipids are modified by enzymes residing in the cell's various organelles 3. Modifications make each compartment's membranes unique in composition & give them distinct identity 4. These modifications are done by the same enzymes that modify secretory proteins that are free in the ER lumen B. Cell membranes are asymmetric; the 2 phospholipid layers (leaflets) have different compositions 1. Asymmetry is initially established in ER as lipids & proteins are inserted preferentially into one layer or the other 2. Asymmetry is maintained while membrane passes through cell by budding & fusion from one compartment to next 3. Components at ER luminal surface are on luminal surfaces of transport vesicles, Golgi cisternae & external (exoplasmic) surface of plasma membrane 4. Similarly, components on ER cytosolic surface maintain their orientation & are ultimately found at internal (cytoplasmic) surface of plasma membrane II. Synthesis of membrane lipids A. Most membrane lipids are produced entirely in ER membrane with following exceptions: 1. Sphingomyelin & glycolipids, the synthesis of which starts in ER & is completed in Golgi complex 2. Some unique mitochondrial/chloroplast membrane lipids (made by enzymes in those membranes) B. Phospholipids are made by integral ER membrane enzymes whose active sites face cytosol 1. Newly synthesized phospholipids are inserted into the outer (cytoplasmic) leaflet of ER membrane 2. Some of the lipids move to inner leaflet aided by flippases (actively translocate them across bilayer) 3. Lipids are carried from ER to Golgi complex & plasma membrane as part of bilayers making up transport vesicle walls C. Membranes of different organelles have markedly different lipid composition (changes made as membrane flows through cell) - what factors contribute to these changes? 1. Conversion of one type of phospholipid to another - most organelles have enzymes that modify lipids already present in membrane (example – phosphatidylserine to phosphatidylcholine) 2. As membranes bud, some phospholipids preferentially included in forming vesicle, others excluded 3. Phospholipid-transfer proteins move specific phospholipids between membrane compartments through aqueous cytosol & may move them from ER to other organelles (mitochondria, chloroplasts) The Endoplasmic Reticulum (ER): Glycosylation in the Rough Endoplasmic Reticulum 330 I. Most proteins made on RER are glycosylated & thus become glycoproteins, whether integral proteins of membrane, soluble lysosomal or vacuolar enzymes or parts of ECM A. Carbohydrate groups – have key roles in function of many glycoproteins (e. g., binding sites in their interactions with other macromolecules); also aid in proper folding of protein to which they are attached 1. Sugar sequences that comprise glycoprotein oligosaccharides are highly specific 2. Sugar sequences from purified glycoprotein are consistent & predictable - how determined? B. How is oligosaccharide sugar sequence assembled? – catalyzed by a family of membrane-bound enzymes (glycosyltransferases) 1. Glycosyltransferases transfer specific monosaccharide from a nucleotide sugar 2. Donor is always a nucleotide sugar - GDP-mannose, GDP-fucose, UDP-galactose, UDP-Nacetylglucosamine; acceptor of transferred sugar is growing end of carbohydrate chain 3. Sequence of sugar transfer during oligosaccharide assembly depends on the sequence of action of glycosyltransferases participating in process 4. Glycosyltransferase sequence, in turn, depends on the location of specific enzymes within the various secretory pathway membranes 5. Thus, sugar arrangement in oligosaccharide chains of a glycoprotein depends on the spatial localization of certain enzymes in this assembly line II. Carbohydrate chains are attached to protein by N-linkages (asparagine N atom) or O-linkages (to serine or threonine O or collagen hydroxylysine residue) of both soluble & integral membrane proteins A. These oligosaccharides differ in average size, sugar composition & path of synthesis & also share properties like their high specificity B. N-linked basal (core) chain segment is assembled on lipid carrier not protein; then transferred as a block to specific asparagine residues of polypeptide as it enters RER by oligosaccharyltransferase 1. Lipid carrier is dolichol phosphate; embedded in membrane (hydrophobic molecule built from >20 isoprene units) & sugars are added one at a time by membrane-bound glycosyltransferases 2. This part of glycosylation process is essentially invariant 3. In mammalian cells, it starts with transfer of N-acetylglucosamine 1-phosphate & then transfer of another N-acetylglucosamine, then 9 mannose & 3 glucose units in a precise pattern 4. This block of 14 sugars is then transferred by oligosaccharyltransferase from dolichol phosphate to nascent polypeptide as it is being translocated into ER lumen III. Mutations that lead to total absence of N-glycosylation cause death of embryos prior to implantation; A. Mutations leading to partial glycosylation pathway disruption in ER also cause serious inherited disorders affecting nearly every organ system B. These diseases are called Congenital Diseases of Glycosylation (CDGs) & they are usually identified through blood tests that detect abnormal glycosylation of serum proteins C. Example: One of these diseases, CDG1b can be managed through a remarkably simple treatment 1. It results from deficiency of the enzyme phosphomannose isomerase (catalyzes conversion of fructose-6-phosphate to mannose-6-phosphate) 2. Its reaction is a crucial reaction in the pathway that makes mannose available for incorporation into oligosaccharides 3. The disease can be managed by giving patients oral supplements of mannose; first tested in boy who was dying from uncontrolled gastrointestinal bleeding (a usual complication of the disease) 4. Within months of taking mannose supplements, the child was living a normal life IV. Some oligosaccharides, especially in lower eukaryotes, are simply the core, but they tend to diversify in more complex organisms (evolution accompanied by diversification of the CHO sequences on proteins) A. After block added, core is modified first in ER with enzymatic removal of 2 of 3 terminal glucose residues by glucosidases B. This sets the stage for an important event in a newly made glycoprotein's life 331 1. During this stage, the glycoprotein is screened by a system of quality control that determines whether or not it is fit to move to the next compartment of the biosynthetic pathway 2. The screening process begins with each glycoprotein, which at this stage contains a single remaining glucose, binding to an ER chaperone (calnexin or calreticulin) 3. Removal of remaining glucose by glucosidase II leads to release of glycoprotein from chaperone C. If folding is incomplete or if protein is misfolded, it is recognized & bound by monitoring enzyme (GT) 1. If GT binds to the glycoprotein, it adds a single glucose back to one of the mannose residues at the exposed end of the recently trimmed oligosaccharide 2. GT recognizes incompletely folded or misfolded proteins because they display exposed hydrophobic residues that are absent from properly folded proteins 3. Once the glucose residue is added, the tagged glycoprotein is recognized by the same chaperones giving it another chance to fold properly 4. After some time with chaperone, the added glucose is removed & GT checks the protein again to see if it has achieved its proper 3D structure (is it partially unfolded or misfolded?) 5. If 3D structure is right, protein continues on its way; if not, glucose is added & process repeats until eventually, the glycoprotein has folded correctly or it remains misfolded & is destroyed The Endoplasmic Reticulum (ER): Mechanisms That Ensure Destruction of Misfolded Proteins I. Misfolded proteins are not destroyed in ER, but are instead transported into cytosol by dislocation A. It remains unclear whether misfolded proteins are dislocated back into cytosol through translocons that brought them into ER or by way of a separate dislocation channel of uncertain identity B. Once in cytosol, misfolded proteins are destroyed in proteasomes, which are protein-degrading machines; this process ensures that aberrant proteins are not transported to other parts of cell 1. But this can have negative consequences; in severe cases of cystic fibrosis, the plasma membrane of epithelial cells is lacking the abnormal protein encoded by the cystic fibrosis gene 2. In these cases, the mutant protein is destroyed by the quality control process & thus fails to reach the cell surface II. Sometimes, misfolded proteins can be generated in ER at a rate faster than they can be exported to the cytoplasm A. The accumulation of misfolded proteins, which is a potentially lethal situation, triggers a comprehensive "plan of action" within the cells known as the unfolded protein response (UPR) B. The ER contains sensors that monitor the concentration of unfolded or misfolded proteins in ER lumen C. One proposal suggests that the sensors are normally kept in an inactive state by molecular chaperones, particularly BiP 1. If circumstances lead to an accumulation of misfolded proteins, the BiP molecules in the ER lumen become "tied up" as a result of their interaction with the misfolded proteins 2. This renders them (the BiP molecules) incapable of inhibiting the sensors; activation of the sensors leads to a multitude of signals that are transmitted into both the nucleus & cytosol 3. This results in the expression of hundreds of different genes whose encoded proteins have the potential to alleviate stressful conditions within the ER D. Among the genes expressed are genes that encode: 1. ER-based molecular chaperones that can help proteins reach the native state 2. Proteins involved in the transport of the proteins out of the ER 3. Proteins involved in the selective destruction of abnormal proteins as described above E. The UPR is more than cell-survival mechanism; it includes the activation of a cell-death pathway 1. It is presumed that the UPR gives the cell an opportunity to relieve itself of the stressful conditions 2. If these corrective measures are unsuccessful, the cell-death pathway is triggered & cell is destroyed 332 From the ER to the Golgi Complex: The First Step in Vesicular Transport I. The exit sites of RER cisternae are typically smooth-surfaced (devoid of ribosomes) & serve as places where the first transport vesicles in biosynthetic pathway are formed II. Trip from ER to Golgi has been visualized in living cells by tagging secretory proteins with green fluorescent protein (GFP) A. After budding from ER membrane, transport vesicles are seen to fuse to each other to form larger vesicles & interconnected tubules in region between ER & Golgi complex B. This region is called ERGIC (endoplasmic reticulum Golgi intermediate compartment) & the vesiculartubular clusters that form there are called VTCs C. Once formed, VTCs move farther away from the ER toward Golgi complex; other studies indicate that this movement occurs along tracks composed of microtubules The Golgi Complex I. Discovered by Camillo Golgi (Italian biologist, 1898) – inventor of new types of staining procedures that he hoped might reveal the organization of nerve cells within the central nervous system A. One stain used solution of silver nitrate applied to tissue that had been soaked in osmium & bichromate 1. Applied stain for several days to cerebellum nerve cells & saw darkly staining reticular network near the cell nucleus; he got the Nobel Prize in part for this discovery in 1906 2. Later seen in other cell types & named Golgi complex; some believed it existed in living cells, others thought it was an artifact (artificial structure formed during preparation for microscopy) 3. For decades, the reality of its existence was the center of a controversy B. Existence confirmed beyond a reasonable doubt when it was clearly identified in unfixed, freeze-fractured cells; it was no artifact II. Characteristic morphology - flattened, disk-like membranous cisternae with dilated rims & associated vesicles & tubules (smooth membranes so found with smooth microsomes) A. Cisternae (typically 0.5 - 1.0 µm dia) arranged in orderly stack like pancakes; curved resembling a shallow bowl; individual Golgi stacks often interconnected to form ribbonlike complex 1. In plants, a single Golgi stack is sometimes called dictyosome B. Usually <8 cisternae are present per stack, but may have a few to several 1000 distinct stacks/cell; depends on cell type 1. Mammalian cell Golgi stacks are interconnected by membranous tubules to form a single, large ribbonlike complex situated adjacent to the cell's nucleus 2. Vesicles seem to bud from a peripheral tubular domain of each cisterna; many vesicles seem to have a distinct protein coat III. Golgi cisternae polarized - cis face (entry face closest to ER); trans face (exit face at opposite end of stack; closer to plasma membrane) A. Golgi complex is divided into several functionally distinct compartments arranged along a cis-trans axis; new materials enter cis face & pass to trans face where they exit Golgi complex 1. cis-most face composed of interconnected network of tubules (cis Golgi network; CGN); CGN & seems to be mostly a sorting station (ships some proteins on further into Golgi, some back to ER) 2. Bulk of Golgi complex consists of a series of large, flattened cisternae divided into 3 regions: the cis cisternae, medial cisternae, trans cisternae 3. Trans-most face has distinct network of tubules & vesicles (trans Golgi network; TGN); also sorting station; proteins placed into different vesicle types (either to membrane or elsewhere in the cell) 333 B. Membranous elements of Golgi complex may be supported mechanically by a peripheral membrane skeleton or scaffold composed of a variety of proteins, including: 1. Members of spectrin, ankyrin, & actin families (these proteins are also present as part of the plasma membrane skeleton) 2. The Golgi scaffold may be linked with motor proteins that direct the movement of vesicles & tubules entering & exiting the Golgi complex 3. A separate group of fibrous proteins form a Golgi "matrix" that plays a key role in the reconstruction of the Golgi complex following mitosis C. Golgi complex composition is not uniform from cis- to trans-end; polarized; differences in composition of membrane compartments (polarization) reflects primary processing plant role 1. Newly synthesized membrane proteins (also secretory & lysosomal proteins) leave the ER & enter the Golgi complex at its cis-face & then pass across the stack to the trans face D. As they move along the stack, proteins originally synthesized in RER are sequentially modified in specific ways; for example: 1. Part of the protein's length may be trimmed by proteolytic enzymes 2. Amino acids may be modified (hydroxylation of lysine & proline residues of a collagen molecule) 3. The protein's carbohydrates are modified by a series of stepwise enzymatic reactions IV. Glycosylation in Golgi complex - synthesis site of most of cell’s complex polysaccharides (animal ECM GAGs; plant cell wall pectins & hemicellulose); key role in glycoprotein/glycolipid CHO assembly A. In ER, glucose residues had just been removed (see above) from the ends of core oligosaccharide of Nlinked CHO chains 1. As newly synthesized soluble & membrane glycoproteins pass though cis & medial Golgi cisternae, most of the mannose residues are also removed from the core oligosaccharides 2. Other sugars are added sequentially by various glycosyltransferases to produce a variety of different oligosaccharides B. In Golgi, as in RER, sequences in which sugars are inserted into oligosaccharides is determined by spatial arrangement of specific glycosyltransferases that contact new proteins as they pass through 1. Sialyltransferase (puts sialic acid at chain terminal position in animal cells) is found in trans end of Golgi stack; expected if new glycoproteins were continually moving toward this part of organelle 2. In ER, a single core oligosaccharide is assembled; in Golgi complex, glycosylation steps can be quite varied, producing carbohydrate domains of remarkable sequence diversity 3. Proteins in RER lack sugars that are normally added in medial & trans Golgi cisternae C. Unlike N-linked oligosaccharides, whose synthesis starts in ER, those attached to proteins by O-linkages are assembled wholly within Golgi complex V. Vesicular transport within Golgi; how do materials move through Golgi? —> 2 contrasting theories A. Cisternal maturation model (up to mid-1980s) – it was accepted that cisternae were transient structures; form at cis face by ER/ERGIC vesicle fusion, travel to trans face & altered along the way 1. Cisternae mature & change in composition as they move through Golgi complex; each cisterna matures into next cisterna along stack (origin of name) 2. Each cisterna was thought to physically move from the cis to the trans end of the stack, changing in composition as it progressed B. New model favored (mid-1980s until late-1990s) – cisternae of Golgi stack remain in place as stable compartments held together by protein scaffold; known as the Vesicular Transport Model 1. Cargo (secretory, lysosomal, membrane proteins) is shuttled through Golgi stack from CGN to TGN in vesicles that bud from one compartment & fuse with neighboring one farther along stack VI. Acceptance of Vesicular Transport Model based largely on the following observations: A. Each of the various Golgi cisternae of stack has distinct resident enzyme population; how could various cisternae have such different properties if each gave rise to next in line as stated by other model? 334 B. Large numbers of vesicles are seen in electron micrographs to bud from rims of Golgi cisternae - James Rothman, et al. (Stanford, 1983) 1. Using cell-free preparations of Golgi membranes, they showed that transport vesicles could bud from one Golgi cisterna & fuse with another Golgi cisterna in vitro 2. Formed basis for hypothesis suggesting that inside cell, cargo-bearing vesicles budded from ciscisternae & fused with cisternae derived from a more trans position in stack VII. Both models still have proponents, but consensus has shifted in past few years back to cisternal maturation model; several major reasons summarized below: A. Cisternal maturation (CM) model envisions a highly dynamic Golgi complex in which major elements of organelle, the cisternae, are continually being formed at the cis face & dispersed at the trans face 1. According to this view, the very existence of the Golgi complex itself depends on the continual influx of transport carriers from the ER & ERGIC 2. As CM model says, when transport carrier formation from ER is blocked either by cell treatment with specific drugs or use of temperature-sensitive mutants, Golgi complex simply disappears 3. When the drugs are removed or the mutant cells are returned to the permissive temperature, the Golgi complex rapidly reassembles as ER-to-Golgi transport is renewed B. Certain materials that are produced in ER & then travel through Golgi complex can be shown to stay within Golgi cisternae & never appear within Golgi-associated transport vesicles 1. Example: fibroblast studies – large complexes of procollagen molecules (extracellular collagen precursors) move from cis cisternae to trans cisternae without ever leaving the cisternal lumen C. Until mid-1990s, it was assumed that transport vesicles always moved in forward (anterograde) direction, from cis origin to trans destination, but new evidence says that…… 1. Some move in backward (retrograde) direction from trans donor to cis acceptor membrane VIII. Revised cisternal maturation model acknowledges a role for transport vesicles, which have been clearly shown to bud from Golgi membranes A. In this model, transport vesicles do not shuttle cargo in anterograde direction, but instead carry resident Golgi enzymes in retrograde direction 1. This model of intra-Golgi transport is strongly supported by electron micrographs showing ultra-thin sections of cultured mammalian cells that were cut from a frozen block 2. Frozen sections were treated with antibodies that were linked to gold particles prior to examination in EM; the antibodies were made against a cargo protein (the viral protein VSVG protein) 3. VSVG molecules were present within cisternae, but absent from nearby vesicles, suggesting that cargo is carried in anterograde direction within maturing cisternae but not in small transport vesicles B. In another experiment, treated gold-labeled antibodies that bind to a Golgi resident protein (the enzyme mannosidase II) —> it was found in both the cisternae & associated vesicles 1. This strongly supports the proposal that these vesicles are utilized to carry Golgi-resident enzymes in a retrograde direction C. The revised cisternal maturation model explains how different Golgi cisternae in a stack can have a unique identity 1. The enzyme mannosidase II removes mannose residues from oligosaccharides & is mostly restricted to medial cisternae 2. It can be recycled backward in transport vesicles as each cisterna moves toward trans end of stack D. Some prominent researchers still argue that cargo can be carried by transport vesicles between Golgi cisternae in an anterograde direction, so the matter is not yet settled The Types of Vesicle Transport and Their Function: Background Information 335 I. Materials carried between membrane compartments by vesicles or other types of membrane-bound carriers, which bud from donor membranes & fuse with acceptor membranes A. Most budding vesicles covered on cytosolic surface by fuzzy, electron-dense layer 1. The dark-staining layer consists of a protein coat formed from soluble proteins that assemble on the donor membrane cytosolic surface at sites where budding takes place 2. Each coated bud pinches off to form a coated vesicle; assembly is initiated by the activation of a small G protein that is specifically recruited to the site 3. Vesicles of similar size & structure can be formed in cell-free systems B. Protein coats have at least two distinct functions: 1. They act as a mechanical device that causes the membrane to curve & form a budding vesicle 2. They provide a mechanism for selecting components (& thus soluble cargo) to be carried by vesicle C. Components selected for transport can include: 1. Cargo to be transported (secretory, lysosomal, & membrane proteins) 2. Machinery required to target & dock the vesicle to an acceptor membrane D. Protein coats are able to make these selections by virtue of their specific affinity for the cytosolic "tails" of integral proteins that reside in the donor membrane E. Vesicle membrane phospholipids also play an important role in selection 1. Phosphate groups can be added to different positions of the sugar ring of the phospholipids phosphatidylinositol (PI) converting them into phosphoinositides 2. The phosphorylated rings of these phosphoinositides reside at the surface of the membrane where they can be recognized & bound by particular proteins 3. Different phosphoinositides are concentrated in different membrane compartments, which helps give each compartment a unique "surface identity" 4. The inner leaflet of the plasma membrane, for example, tends to contain elevated levels of PI(4,5)P 2, which plays an important role in recruitment of proteins like clathrin to endocytosis sites 5. A lipid species like PI(4,5)P2 can have a dynamic regulatory role because it can be rapidly formed & destroyed by enzymes that are localized at particular places & times within the cell II. Several distinct classes of coated vesicles have been identified - distinguished by the proteins that make up the coat, their appearance in EM, & their role in cell trafficking; three are the best-studied: A. COPII-coated vesicles - move materials forward from ER to ERGIC (intermediate compartment between ER & Golgi) & Golgi complex; COP is acronym for coat proteins B. COPI-coated vesicles - move materials in retrograde direction from ERGIC & Golgi stack backward toward ER 1. Also thought to transport materials through Golgi from cis to trans face 2. May play role in trafficking from ER to Golgi, from TGN to cell membrane, between compartments of endocytic pathway C. Clathrin-coated vesicles - move materials from TGN to endosomes, lysosomes & plant vacuoles 1. Also move materials from plasma membrane to cytoplasmic compartments along endocytic pathway 2. Also implicated in trafficking from endosomes & lysosomes COPII-Coated Vesicles: Transporting Cargo from the ER to the Golgi Complex I. COPII-coated vesicles are the most recently discovered & mediate the first leg of journey through the biosynthetic pathway from ER to ERGIC & CGN A. COPII coat contains a number of proteins first found in mutant yeast cells that could not transport materials from ER to Golgi; homologous proteins found in coats of vesicles budding from mammalian cell ER B. Antibodies to COPII-coat proteins block vesicle budding from ER membranes but have no effect on movement of cargo at other stages in the secretory pathway 336 II. COPII-coated vesicles are thought to be able to select & concentrate certain components that they transport A. ER integral membrane proteins are selectively captured because they interact specifically with COPII proteins of vesicle coat; several types of membrane proteins are included in this group: 1. Enzymes that act at later stages of biosynthetic pathway, like glycosyltransferases of Golgi complex 2. Membrane proteins involved in docking & fusion of the vesicle with the target compartment 3. Membrane proteins that bind soluble cargo (secretory proteins), e. g., membrane protein, ERGIC-53, that binds to mannose residues found on oligosaccharides of certain secretory proteins in ER B. Example: ERGIC-53 mutations have been linked to an inherited bleeding disorder; people with the disorder fail to secrete certain coagulation factors that promote blood clotting III. Interaction between membrane proteins (like ERGIC-53) & the COPII-coat is mediated by signal sequences in the cytosolic tails of the membrane proteins A. ERGIC-53, for example, is recognized by 2 neighboring phenylalanines in its cytosolic tail B. Other types of soluble cargo are not selected at this stage & are present at the same concentration in the budding vesicle as in ER lumen 1. Proteins that become enclosed in vesicles but are not specifically selected for inclusion are said to move by bulk flow 2. Some of the integral ER membrane proteins may also become trapped in budding vesicles & transported through secretory pathway to plasma membrane by bulk flow IV. Among COPII coat proteins is a small G protein (Sar1); it is recruited specifically to ER membrane; like other G proteins Sar1 plays regulatory role, here, it starts vesicle formation & regulates vesicle coat assembly A. First, Sar1 is recruited to the ER membrane in the GDP-bound form & is induced to exchange its GDP for a GTP molecule (Step 1) B. Upon binding GTP, Sar1 undergoes a conformational change that causes its N-terminal helix to insert itself into the cytosolic leaflet of the ER bilayer (Step 2) 1. This event has been shown to bend the lipid bilayer, which is an important step in the conversion of a flattened membrane into a spherical vesicle 2. Membrane bending is probably aided by a change in packing of the lipids that make up the 2 leaflets of the bilayer C. In Step 3, Sar1–GTP has recruited 2 additional polypeptides of the COPII coat, which bind as a "bananashaped" dimmer 1. Because of its curved shape, this dimer provides additional pressure on the membrane surface to help it further bend into a curved bud D. In Step 4, the remaining subunits of the COPII coat bind to the membrane, and the bud is separated from the ER membrane in the form of a COPII-coated vesicle E. Before the coated vesicle can fuse with a target membrane, the protein coat must be disassembled and its components released into the cytosol 1. Disassembly is triggered by hydrolysis of the bound GTP to produce a Sar1-GDP subunit, which has decreased affinity for the vesicle membrane 2. Dissociation of Sar1-GDP from the membrane is followed by the release of the other COPII subunits COPI-Coated Vesicles: Transporting Escaped Proteins Back to the ER I. COPI-coated vesicles were first identified in experiments where cells were treated with GTP analogues (molecules with structures similar to GTP) that cannot be hydrolyzed (unlike GTP) A. In the presence of these analogues, COPI-coated vesicles accumulated within the cell & could be isolated from homogenized cells by density gradient centrifugation 1. They accumulate in presence of analogue because (like COPII coat) their coat contains a small GTPbinding protein (ARF1), whose bound GTP must be hydrolyzed before the coat can disassemble 2. ARF1 (adenosylation ribose factor) is 1 of 8 distinct proteins to make up complete COPI coat 337 B. COPI-coated vesicles have been most clearly implicated in the retrograde transport of proteins including the movement of: 1. Golgi-resident enzymes in a trans-to-cis direction (like mannosidase II) 2. ER-resident enzymes from the ERGIC & the Golgi complex back to the ER C. Whether or not COPI-coated vesicles are involved in anterograde and/or retrograde transport between Golgi cisternae remains a matter of controversy II. Retaining & retrieving resident ER proteins – if vesicles continually bud from membrane compartments, how does each compartment retain its unique composition? A. What determines whether a particular ER membrane protein stays in ER or goes on to Golgi complex? studies suggest proteins are maintained in an organelle by a combination of 2 mechanisms: 1. Retention of resident molecules that are excluded from transport vesicles, and 2. Retrieval of escaped molecules back to the compartment in which they normally reside B. Mechanism of retention in particular membrane is not understood 1. Retained proteins may become part of complexes that are too large to be incorporated into a budding transport vesicle or…. 2. Membranes may have different domains with dissimilar chemical composition & physical properties (membrane microheterogeneity) a. It is possible that transported membrane proteins must reside in a particular ER membrane domain that can be captured by the COPII coat, allowing retention of other proteins III. Retrieval of escaped proteins is better understood - proteins that normally reside in ER (in lumen & membrane) have short amino acid sequences at C-terminus that serve as retrieval sequences A. Ensures their return to ER if they are carried forward accidentally in transport vesicle to ERGIC or Golgi complex B. Retrieval of "escaped" ER proteins from these compartments is accomplished by specific receptors that capture the molecules & return them to the ER in COPI-coated vesicles C. Soluble proteins of ER lumen (protein disulfide isomerase & molecular chaperones that facilitate folding) typically possess the retrieval signal "lys-asp-glu-leu" [KDEL in single letter nomenclature] 1. Soluble ER proteins with KDEL signal are recognized & bound by an integral membrane protein, the KDEL receptor, whose cytosolic tail binds to COPI coat, ensuring their return to ER 2. If KDEL sequence is deleted from ER protein, the ER proteins are not recovered & brought back to the ER compartment, but instead are carried forward through the Golgi complex 3. If a scientist engineers a lysosomal or secretory protein in cell to have an added KDEL C-terminus, the protein is returned to ER rather than going to its proper destination D. ER integral membrane proteins (like SRP receptor) have a different retrieval signal at C-terminus (usually KKXX, where K is lysine & X is any amino acid); it binds to COPI coat & ensures return E. Each biosynthetic pathway compartment may have its own unique retrieval signals; this explains the maintenance of unique protein complements in each one despite constant in/out vesicle movement Beyond the Golgi Complex: Sorting of Lysosomal Proteins at the TGN I. How does particular protein synthesized in ER get targeted toward particular cellular destination? A. Cell must be able to distinguish among the various proteins it manufactures – example: pancreatic cell 1. Must segregate newly made digestive enzymes (secreted into duct), from newly made cell-adhesion molecules (ultimately reside in plasma membrane), from lysosomal enzymes destined for lysosomes 2. So the cell sorts proteins destined for different sites into different vesicles, determining destination B. Protein sorting occurs in the last of the Golgi compartments, the trans Golgi network (TGN), which functions as a major branch point in the movement of materials along the secretory pathway 1. The TGN is the site of assembly of clathrin-coated vesicles 2. Clathrin coats mediate cargo sorting at TGN & clathrin-coated vesicles carry hydrolytic enzymes & membrane proteins from there to endosomes, lysosomes & plant vacuoles 338 II. Lysosomal protein sorting & transport - made on membrane-bound RER ribosomes, carried to cis Golgi cisternae with other protein types; this is the best understood post-Golgi pathway (for lysosomal enzymes) A. Once in Golgi cisternae, soluble lysosomal enzymes recognized by enzymes catalyzing 2-step addition of phosphate group to certain N-linked CHO chain mannose sugars 1. Unlike other glycoproteins sorted at the TGN, lysosomal enzymes possess phosphorylated mannose residues, which act as recognition signals 2. This mechanism of protein sorting was discovered through studies on human cells that lacked one of the enzymes involved in phosphate addition B. Lysosomal enzymes with mannose 6-phosphate signal are recognized & captured by mannose-6phosphate receptors (MPRs; integral membrane proteins that span the Golgi membranes) C. Lysosomal enzymes are transported from TGN in clathrin-coated vesicles; coats of the vesicles contain: 1. An outer honeycomblike lattice composed of the protein clathrin, which forms a structural scaffold 2. An inner shell made of protein adaptors that cover the vesicle membrane surface facing the cytosol; in molecular biology, an adaptor is a molecule that physically links 2 different types of materials D. Lysosomal enzymes are escorted from the TGN by a family of adaptor proteins called GGAs 1. Each GGA molecule has several domains, each capable of grasping a different protein involved in vesicle formation 2. The outer ends of GGA adaptors bind to clathrin molecules, holding the clathrin scaffolding onto the surface of the vesicle 3. On their inner surface, GGA adaptors bind to sorting signals in the cytosolic tails of the mannose 6phosphate receptors 4. The MPRs, in turn, bind to soluble lysosomal enzymes within the vesicle lumen 5. As a result of these interactions with GGA adaptors, MPRs in TGN membrane & lysosomal enzymes within TGN lumen become concentrated into clathrin-coated vesicles E. As with COPI/COPII vesicle formation, clathrin-coated vesicle production starts with recruitment to the membrane of small GTP-binding protein (ARF1), which sets the stage for binding of other coat proteins F. Once the vesicle has budded from the TGN, the clathrin coat is lost & the uncoated vesicle proceeds to its destination, which may be an endosome, lysosome or plant vacuole G. Once the vesicle reaches its destination organelle, the MPRs dissociate from the lysosomal enzymes & return to the TGN for another round of lysosomal enzyme transport Beyond the Golgi Complex: Sorting and Transport of Non-Lysosomal Proteins I. Membrane proteins destined for plasma membrane & secretory proteins destined for export from the cell are also transported from TGN, but the mechanisms are poorly understood A. Recent model – membranous carriers are produced as the TGN fragments into vesicles & tubules of various sizes; this fits with cisternal maturation model 1. Cisternal maturation model suggests that Golgi complex cisternae move continually toward TGN, where they would have to disperse to allow continued maturation of Golgi stack 2. Proteins that are discharged from the cell by a process of regulated secretion (digestive enzymes, hormones) are thought to form selective aggregates 3. These aggregates eventually become contained in large, densely packed secretory granules & are apparently trapped as secretory granules bud from the rims of the trans Golgi cisternae & TGN 4. Secretory granules are then stored in the cytoplasm until their contents are released after stimulation of the cell by a hormone or nerve impulse II. The targeted delivery of integral proteins to the plasma membrane appears to be based largely on sorting signals in the cytoplasmic domains of the membrane proteins A. In polarized cells, membrane proteins destined to reside in the apical portions of the plasma membrane contain different sorting signals from those destined for the lateral or basal portion 339 B. Plasma membranes of nonpolarized cells (fibroblasts, white blood cells) may not require special sorting signals 1. Such proteins may simply be carried from the TGN to the cell surface in vesicles of the constitutive secretory pathway Beyond the Golgi Complex: Targeting Vesicles to a Particular Compartment: Background I. Vesicle fusion requires specific interactions between different membranes A. Vesicles from ER fuse with ERGIC or cis Golgi network & not with a trans cisterna B. Selective fusion occurs & is one factor that helps ensure a highly directed flow through the membranous compartments of the cell II. The way in which cells target vesicles to specific compartments is not well understood but vesicles are thought to have specific proteins in their membranes governing their movements & fusion potential III. Summary of the steps between vesicle budding & vesicle fusion is needed to understand the nature of the proteins in vesicle membranes controlling vesicle movement & fusion Targeting Vesicles to a Particular Compartment: Summary of Steps Between Vesicle Budding and Fusion I. Movement of vesicle toward the specific target compartment A. Vesicles must sometimes move large distances through cytoplasm before reaching its eventual target; probably directed by microtubules B. Microtubules act like railroad tracks carrying cargo containers along a defined pathway to a predetermined destination II. Tethering vesicles to the target compartment – microscope studies indicate that vesicles are often tethered to a presumed target compartment like Golgi cisterna, by extended, fibrous proteins A. Tethering may be an early stage in process of vesicle fusion that requires specificity between vesicle & target compartment B. Much of this specificity may be conferred by a family of small GTP-binding proteins (Rabs); 1. With >60 different Rab genes identified in humans, these proteins constitute the most diverse group of proteins involved in membrane trafficking 2. More importantly, different Rabs become associated with different membrane compartments 3. This preferential localization gives each compartment a unique surface identity, which is required to recruit the proteins involved in targeting specificity 3. In their GTP-bound state, Rabs are thought to recruit specific cytosolic tethering proteins to specific membrane surfaces III. Docking vesicles to the target compartment – at some point during the process leading to vesicle fusion, membranes of vesicle & target compartment become tightly apposed to one another A. This is result of interaction between the cytosolic regions of integral proteins of the 2 membranes 1. The key proteins that engage in these interactions are called SNAREs & they constitute a family of membrane proteins whose members are localized to specific subcellular compartments 2. SNAREs vary a lot in structure & size, but all of them contain a segment in their cytosolic domain (a SNARE motif) consisting of 60 – 70 amino acids that form a complex with another SNARE motif B. SNAREs are divided functionally into 2 categories: v-SNAREs (incorporated into transport vesicle membranes during budding) & t-SNAREs (located in target compartment membranes) C. The best-studied SNAREs are those that mediate docking of synaptic vesicles with the presynaptic membrane during the regulated release of neurotransmitters 340 1. Presynaptic nerve cell membrane contains 2 t-SNAREs: syntaxin & SNAP-25, while the synaptic vesicle membrane contains a single v-SNARE, synaptobrevin 2. As synaptic vesicle & presynaptic membranes approach one another, the SNARE motifs of t- & vSNARE molecules from apposing membranes interact with one another to form 4-stranded bundles 3. Each bundle consists of 2 -helices donated by SNAP-25 & 1 -helix donated by both syntaxin & synaptobrevin 4. Together, these parallel -helices form a tightly interwoven complex that pulls the two apposing lipid bilayers into very close association 5. The formation of similar 4-stranded helical bundles occurs among other SNAREs at other sites throughout the cell, wherever membranes are destined to fuse D. Interestingly, the SNAREs of synaptic vesicle & presynaptic membranes are targets of two of the most potent bacterial toxins, those responsible for botulism & tetanus 1. These deadly toxins act as proteases, whose only known substrates are SNAREs 2. Cleavage of the neuronal SNAREs blocks the release of neurotransmitters, which causes paralysis IV. Fusion between vesicle & target membranes A. When artificial lipid vesicles (liposomes) containing purified t-SNAREs are mixed with liposomes containing a purified v-SNARE, the two types of vesicles fuse with one another but not themselves 1. This finding indicates that interactions between v- & t-SNAREs are capable of pulling two lipid bilayers together with sufficient force to cause them to fuse 2. Evidence suggests that while an interaction between v- & t-SNAREs is required for fusion, it is not sufficient alone to bring about fusion within a cell B. One view regarding the regulated secretion of neurotransmitter molecules 1. The 4-stranded SNARE bundle remains locked in an inactive conformation 2. Vesicles at this stage remain docked at the membrane & ready to discharge their contents almost instantaneously once they receive an activating signal in the form of a rise in Ca 2+ concentration 3. Regardless of how it is regulated, once membrane fusion occurs, the SNAREs that previously projected from separate membranes become situated in the same membrane 4. Dissociation of 4-stranded SNARE complex is achieved by doughnut-shaped, cytosolic protein called NSF that attaches to the SNARE bundle &, using energy from ATP hydrolysis, twists it apart C. How is specificity of this interaction determined? – current consensus is that the ability of a particular vesicle & target membrane to fuse is determined by the specific combination of interacting proteins 1. The proteins include tethering proteins, Rabs & SNAREs; that can be assembled at that site in cell 2. Taken together, these multiple interactions between several types of proteins provide a high level of specificity Exocytosis: The Terminal Stage of Secretion I. Best-studied examples of vesicle fusion are the regulated fusion of secretory or synaptic vesicles with the plasma membrane A. In these cases, membrane fusion produces opening through which vesicle (granule) contents are released into extracellular space B. This process of membrane fusion & content discharge is called exocytosis; it is usually triggered by a local increase in calcium ion concentration 1. The arrival of a nerve impulse at neuron terminal knob leads to an increase in Ca 2+ influx & subsequent neurotransmitter discharge by exocytosis 2. In this case, fusion in neuron is mediated by calcium-binding protein (synaptotagmin) found in synaptic vesicle membrane 3. In other types of cells, exocytosis is usually triggered by Ca2+ release from cytoplasmic stores 4. Injection of Ca2+ solutions into secretory cells leads to wholesale exocytosis of secretory product II. Steps in exocytosis – not well understood 341 A. Cell & vesicle membrane contact mediated by fusion proteins within & on membrane surface; proteins thought to create close-range contact between membranes destined to interact & fuse B. Contact between the cell & vesicle membranes may lead to the formation of a small, protein-lined fusion pore that rapidly dilates to form opening for discharge C. Regardless of mechanism, when a cytoplasmic vesicle fuses with the plasma membrane: 1. The luminal surface of vesicle membrane becomes part of outer surface of plasma membrane and 2. The cytosolic surface of vesicle membrane becomes part of the inner surface of plasma membrane Lysosomes I. Lysosome morphology & contents – typically contain at least 50 different hydrolytic enzymes made in RER & targeted for lysosomes; lysosomes are an animal cell's digestive organelles A. Lysosomal enzymes taken together can hydrolyze virtually every type of biological macromolecule, resulting in low MW products that can be transported across the lysosomal membrane into cytosol B. All of the enzymes have pH optimum at acid pH (acid hydrolases) suited to the low pH of the lysosomal compartment; lysosome interior pH is ~ 4.6 1. The high internal proton concentration is maintained by a proton pump (transporter; an H+-ATPase) present in the lysosome's boundary membrane 2. Lysosmal membranes also contain a variety of highly glycosylated integral proteins; the carbohydrate chains form a lining that may shield membrane from attack by enclosed enzymes C. Lysosomal morphology is neither distinctive nor uniform, although they house a predictable collection of enzymes; they are dynamic organelles capable of rapid fusion & fission 1. Range in size from relatively large (>1 µm diameter) to very small vesicles (25 - 50 nm diameter) 2. Can be irregular in shape & of variable electron density (like those in Kupffer cell, a phagocytic cell in the liver that engulfs aging red blood cells) 3. Hard to identify on morphological basis alone; identifying trait is presence of acid phosphatase (assay produces lead phosphate product visible in EM) II. Lysosomal functions A. Materials brought into cell (protozoa, macrophages, neutrophils) from extracellular environment are enzymatically broken down; resulting nutrients cross membrane into cytosol; best-studied function 1. Many single-celled organisms ingest food particles, which are disassembled in lysosome 2. In mammals, phagocytic cells (macrophages, neutrophils) act as scavengers, ingesting debris & potentially dangerous microorganisms; highly phagocytic cells may have up to 1000 lysosomes 3. Ingested bacteria are usually inactivated by low pH & then digested enzymatically; some are not 4. Peptides made by the above process are posted on cell surface; they alert immune system to presence of foreign agent B. Fertilization - sperm head contains specialized (modified) lysosome (acrosome), which contains typical lysosomal enzymes; unusual because lysosomal enzymes are active outside the cell 1. As sperm nears egg, acrosome membrane fuses with sperm plasma membrane, releasing stored enzymes that digest egg outer covering 2. Leaves hole through which advancing sperm can reach egg surface C. Organelle turnover (autophagy) – regulated destruction of cell's own organelles & their replacement 1. During process, organelle (e. g., mitochondrion) is surrounded by a double membrane derived from an ER cisterna; outer membrane then fuses with lysosome to produce autophagolysosome 2. In the autophagolysosome, the enclosed organelle is degraded & the breakdown products are made available to the cell 3. It is calculated that 1 mitochondrion undergoes autophagy about every 10 min or so in a mammalian liver cell 342 4. If nutrient supply drops, autophagy rate increases to provide missing nutrients & thus energy; cell cannibalizes its own organelles to acquire energy to maintain life D. In recent years, autophagy has also been shown to help protect an organism against intracellular threats ranging from abnormal protein aggregates to invading bacteria 1. Autophagy may even serve as a pathway leading to the programmed death of malignant cells E. Once digestive process in autophagolysosome is completed, organelle is called residual body 1. Depending on cell type, residual body contents may be eliminated from cell by exocytosis or retained within cytoplasm indefinitely as lipofuchsin granule 2. Lipofuchsin granules rise in number with age of individual; accumulation is particularly evident in long-lived cells (neurons) where granules are considered a major characteristic of aging process Plant Cell Vacuoles I. A single, membrane-bound, fluid-filled central vacuole occupies up to 90% of cell volume; they have a wide spectrum of essential functions II. Functions of plant cell vacuoles A. Temporary storehouse for many cell solutes & macromolecules (ions, sugars, amino acids, proteins, polysaccharides) B. May also store a host of toxic compounds (cyanide-containing glycosides & glucosinolates) 1. Some are part of chemical weapon arsenal released when cell is injured by herbivore or fungus or 2. Some are byproducts of metabolic reactions (used to isolate them from rest of cell into vacuole since plants have no excretory system, unlike animals) - some, like digitalis, important clinically C. Generates high turgor pressure that pushes outward against cell wall & maintains cell shape 1. Has high osmotic pressure, since ions pumped into vacuolar compartment by proteins (active transport systems) in membrane (tonoplast) bounding it 2. The ion concentration attained is much higher than that in cytoplasm or extracellular fluid 3. Because of vacuole's high ion concentration, H2O osmoses through tonoplast & into vacuole 4. Hydrostatic (turgor) pressure provides mechanical support for plant soft tissues & provides force needed to stretch cell wall during cell growth D. Sites of intracellular digestion, similar to animal lysosomes; lysosomes are absent in plants 1. Plant vacuoles have some of the same acid hydrolases as found in lysosomes & low pH 2. Low pH maintained by V-type H+-ATPase in tonoplast that pumps protons into vacuolar fluid 3. Vacuoles are also endpoint in cell’s biosynthetic pathway; follow same basic path as lysosome proteins (RER —> through Golgi —> sorted at Golgi trans face —> targeted to vacuole) The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior Background Information and Overview I. Cells must take in materials that are too large to penetrate the membrane regardless of its permability properties & recycle proteins that reside in plasma membrane to cell's internal compartments A. Both of these requirements are met by the endocytic pathway in which segments of the plasma membrane invaginate to form cytoplasmic vesicles that are transported to cell interior B. Two separate processes of uptake of extracellular materials into cytoplasmic vesicles, which occur by different mechanisms – endocytosis & phagocytosis 1. Endocytosis – primarily a process by which the cell internalizes cell surface receptors & bound extracellular ligands (uptake of fluid, dissolved solutes & suspended macromolecules) 2. Phagocytosis – the uptake of particulate matter II. Terminology has changed in recent years; in 1963, C. de Duve introduced the term endocytosis to include ingestion of particles (phagocytosis) & uptake of fluid & solutes (pinocytosis) 343 A. Since it has become clear that phagocytosis & pinocytosis are fundamentally different activities, the term pinocytosis is being used less often B. For example, phagocytic vesicles usually ~10X larger than endocytic ones (1 - 2 µm vs. 0.1 - 0.2 µm in dia) C. Endocytosis is now employed to describe the uptake of both fluid & dissolved or suspended molecules; endocytosis is distinguished from phagocytosis The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior – Introduction to Endocytosis I. Endocytosis - uptake of fluid, dissolved solutes, suspended macromolecules; divided into 2 broad categories: bulk phase & receptor-mediated endocytosis A. Bulk phase endocytosis (also known as pinocytosis) – nonspecific uptake of extracellular fluids without recognition by membrane 1. Any molecules (large or small) that happen to be present in enclosed fluid are taken into cell as well 2. Visualized by adding substance to culture medium (dye lucifer yellow; enzyme horseradish peroxidase); taken up non-specifically 3. Occurs continually in certain cell types where it may function primarily to convert plasma membrane into cytoplasmic membrane; keeps cell from accumulating too much plasma membrane 4. This conversion is required in cells that have been engaged in secretion & have had large numbers of secretory vesicles fuse with the plasma membrane 5. Also removes portions of plasma membrane & may function primarily in the recycling of membrane between the cell surface & interior compartments B. Receptor-mediated endocytosis (RME) – brings about uptake of specific extracellular macromolecules (ligands) that bind to receptors on external plasma membrane surface II. Rate of both processes can be remarkably rapid - up to half membrane surface can be internalized in as little as 30 min A. Despite rapid inward movement of plasma membrane, there is no shrinkage of cell surface B. Nor is there any immediate need for synthesis of new membrane components C. Membrane is simply cycled between surface & cell interior so that membrane is added to surface as fast as it is removed; exocytosis rate equals that of endocytosis (membrane is recycled) The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior – Receptor-Mediated Endocytosis and the Role of Coated Pits I. RME provides means for selective & efficient uptake of macromolecules that may be present at relatively low concentrations in extracellular fluid A. Cells have receptors for the uptake of many different types of ligands (hormones, growth factors, enzymes, plasma proteins) 1. Substances that enter cell by RME bind receptors that collect in specialized areas of plasma membrane (coated pits) 2. Receptors are concentrated in coated pits to 10 - 20X that in rest of membrane B. Coated pits – membrane surface sites that are indented & covered on cytoplasmic face by bristly, electron dense protein layer containing clathrin & adaptors 1. Clathrin is the same protein in clathrin-coated vesicles formed at TGN 2. Coated pits invaginate into cytoplasm & then pinch free of plasma membrane to form coated vesicles II. Structure of coat – when viewed from its cytoplasmic surface, bristly coat appears to consist of network of polygons (hexagons & pentagons) resembling honeycomb; explains formation of coat A. Geometric construction of coat is derived from structure of its clathrin building blocks 344 1. Each clathrin molecule consists of 3 heavy & 3 light chains joined together at the center to form a 3legged assembly (triskelion) B. Triskelions within the clathrin scaffold of a coated vesicle are found in an overlapping arrangement 1. Each leg of a clathrin triskelion extends outward along two edges of a polygon 2. The clathrin molecules overlap in such a way that each vertex of a polygon contains a center of one of the component triskelions III. Like clathrin-coated vesicles budding from TGN, coated vesicles formed during endocytosis also contain a layer of adaptors situated between clathrin lattice & vesicle surface facing the cytosol A. The best-studied adaptor operating in connection with clathrin-mediated endocytosis is AP2 B. Unlike GGA adaptors used at TGN (consisting of a single subunit with several domains), AP2 adaptors incorporated into vesicles budding from cell membrane have multiple subunits with different functions 1. The µ subunit of AP2 adaptors engages cytoplasmic tails of specific membrane receptors leading to concentration of the selected receptors (& bound cargo molecules) into emerging coated vesicle 2. In contrast, AP2 adaptor -adaptin subunit binds & recruits clathrin molecules of overlying lattice 3. 3-legged clathrin triskelions overlap within vesicle coat wall, & the clathrin lattice & adaptors interact C. Unlike COPI & COPII vesicles, which have relatively simple construction, clathrin-coated vesicles have upwards of 2 dozen different accessory proteins forming dynamic network of interacting molecules 1. These proteins have poorly understood roles in cargo recruitment, coat assembly, membrane invagination, interaction with cytoskeletal components, vesicle release & membrane uncoating 2. Best-studied accessory protein is dynamin IV. Dynamin is a large GTP-binding protein that is required for the release of a clathrin-coated vesicle from the membrane on which it forms A. Dynamin self-assembles into a helical collar around the neck of an invaginated coated pit, just before it pinches off from the membrane B. Hydrolysis of bound GTP by the polymerized dynamin molecules is thought to induce a conformational change in the dynamin helix that severs coated vesicle from the plasma membrane 1. Some think that dynamin thus acts as an enzyme that can utilize GTP's chemical energy to generate mechanical forces – this model has considerable experimental support 2. Another model suggests that dynamin acts more like other G proteins by switching on the activity of a separate effector protein that severs the vesicle C. Within a minute of its formation, the coated endocytic vesicle loses its clathrin coat & becomes a smoothsurfaced vesicle that enters the endocytic pathway The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior Overview I. Molecules taken into a cell by endocytosis are routed through a well-defined endocytic pathway II. 2 different types of receptors are subjected to endocytosis A. Housekeeping receptors – responsible for uptake of materials that will be used by cell; best-studied examples are transferrin & LDL receptors; mediate delivery to cells of iron & cholesterol, respectively 1. Endocytosis of these receptors leads typically to the delivery of the bound materials (like iron & cholesterol) to the cell & return of the receptor to the cell surface for additional rounds of uptake B. Signaling receptors – responsible for binding extracellular ligands that carry messages that change cell activities; these ligands (hormones like insulin; growth factors like EGF) do not actually enter cell 1. Instead, they bind to the surface receptor & signal a physiological response inside the cell 2. Their endocytosis leads typically to destruction of receptor (receptor down-regulation), which has the effect of reducing the cell's sensitivity to further stimulation by the hormone or growth factor 345 3. Receptor down-regulation is a mechanism by which cells regulate their ability to respond to extracellular messengers 4. Signaling receptors are usually marked for endocytosis & subsequent destruction by the covalent attachment of a "tag" to the cytoplasmic tail of the receptor while it resides at the cell surface 5. The tag is a small molecule (ubiquitin), which is added enzymatically; membrane proteins that are not normally subjected to endocytosis are internalized if they are made to carry an added ubiquitin III. Endocytic pathway begins with a dynamic network of tubules & vesicles known collectively as endosomes A. Endosome lumen fluid is acidified due to activity of endosome membrane H+-ATPase (H+ pump) B. Endosomes are divided into 2 distinct classes - distinguished from one another on basis of buoyant density (allows them to be isolated in different fractions on density gradient), pH, protein composition 1. Early endosomes - typically located near peripheral region of cell 2. Late endosomes - typically located in more interior part of cell, closer to nucleus C. Late endosomes may contain considerable amounts of internal membrane that arises from inward invaginations of the boundary membrane 1. These internal vesicles often contain plasma membrane proteins on the path to destruction IV. Receptors taken up by endocytosis are transported in endocytic vesicles to an early endosome, which serves as a sorting station that directs different types of receptors & ligands along different pathways A. Housekeeping receptors dissociate from their bound ligands in the acidified endosomal environment 1. The receptors are then concentrated into the specialized tubular compartments of the early endosome, which represent recycling centers 2. Vesicles budding from these tubules carry receptors back to plasma membrane for additional rounds of endocytosis B. In contrast, released ligands (e.g., LDL) become concentrated into a sorting compartment before being dispatched to a late endosome & ultimately to a lysosome, where final processing occurs C. Signaling receptors previously marked with ubiquitin tags do not recycle back to membrane, but instead are sent on to late endosomes & lysosomes where they will ultimately be destroyed V. Steps along endocytic pathway from an early endosome to a lysosome have been described in various ways by different researchers working on different cells A. Several reports – transfer of materials from early to late endodomes occurs by means of specialized carrier vesicles, often referred to as multivesicular bodies (MVBs) 1. Their name derives from fact that they are typically packed with internal vesicles 2. Internal vesicles arise as inward-directed invaginations from the boundary membrane of carrier 3. Internal vesicle membranes contain receptors & other membrane proteins ubiquinated at cell surface 4. This suggests that ubiquitin serves as sorting signal that initially causes protein to be internalized & subsequently causes the protein to end up as part of MVB internal vesicles 5. MVBs move deeper into cell where they either fuse with or mature into late endosomes 6. Either way, late endosomes typically contain considerable amounts of internal membrane that is derived from MVB internal vesicles B. Molecules that travel along endocytic pathway in a late endosome are ultimately directed to a lysosome, the terminal compartment of the endocytic pathway; this movement occurs by 2 major routes 1. Maturation of late endosomes into lysosomes – in addition to getting material from early endosomes, late endosomes get newly made lysosomal enzymes from TGN (carried by receptors) 2. Fusion of late endosomes with preexisting lysosomes C. Once in a lysosome, membrane receptors & other macromolecules are destroyed, but transported materials like cholesterol are typically processed for delivery to the cytosol 1. Once they have delivered their cargo to late endosomes, receptors that carried lysosomal enzymes to the late endosomes are recycled back to the TGN for additional rounds of transport 346 The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior – LDLs and Cholesterol Metabolism I. First & best-studied example of receptor-mediated endocytosis is the mechanism that provides animal cells with exogenous cholesterol A. Cholesterol is a hydrophobic molecule; it serves as a precursor to steroid hormones & an essential part of plasma membrane in animals; can't be free in blood B. Cholesterol is transported through blood as part of huge lipoprotein complexes (like low density lipoprotein; LDL) 1. Each LDL particle has central core (~1500 cholesterols esterified to long-chain fatty acids) 2. Core is surrounded by single layer of phospholipids & a single copy of a large protein, apolipoprotein B-100, which binds specifically to LDL receptors on cell surfaces II. LDL receptors found mostly in liver cells; transported to cell's plasma membrane where wait for LDL A. LDL receptors are concentrated in coated pits even in absence of ligand; thus, they are in plasma membrane & ready to take up blood-borne lipoproteins, if they should become available 1. When it passes membrane, LDL binds to coated pit; the pit invaginates forming coated vesicle, the clathrin coat disassembles, & LDL receptors are recycled back to plasma membrane 2. LDL particles go to lysosomes; protein component is degraded & cholesterol is released for use by cell (membrane assembly or other metabolic processes, like steroid hormone production) B. People with a rare inherited disorder (Niemann-Pick type C disease) lack one of the proteins required to transfer cholesterol out of lysosomes 1. The resulting accumulation of cholesterol in these organelles leads to nerve degeneration & death in early childhood III. LDL & atherosclerosis (narrowing of major arteries) A. LDL blood levels have been related strongly to the development of atherosclerosis B. Recent studies suggest that atherosclerosis results from a chronic inflammatory response that is initiated by the deposition of LDL within the inner walls of the vessels 1. LDL deposition leads to development of plaques on artery walls 2. Plaques reduce blood flow through vessel & act as sites for formation of blood clots (can completely block flow) 3. Clots that block coronary arteries are leading cause of myocardial infarction (heart attack) C. LDL-lowering drugs (statins; pravastatin or lovastatin) - inhibit a key cholesterol synthesis enzyme, HMG CoA reductase; lowers blood cholesterol & heart attack frequency 1. Cells make less cholesterol so must take up more from blood 2. Make more LDL receptors so more LDL taken up & less cholesterol is thus present in blood 3. When blood cholesterol levels are low, the frequency of heart attack is reduced IV. HDLs (high-density lipoproteins), in addition to LDLs, transport cholesterol in blood; similar in construction, but have different protein (apolipoprotein A-1) & play different physiological role in body A. LDL primarily carries cholesterol molecules from liver, where they are synthesized & packaged, through blood to body cells; HDL carries excess cholesterol from body's cell membranes to liver 1. Excess cholesterol is transported out of body cell plasma membranes directly to circulating HDL particles 2. When HDL gets to liver, it is endocytosed & cholesterol is excreted as part of bile 3. HDL is often referred to as the "good cholesterol" B. While high blood LDL levels lead to heart disease; high blood HDL levels are associated with lowered risk (good for heart; it seems to remove cholesterol from blood), but things are not this simple 347 1. The enzyme cholesteryl ester transfer protein (CETP) transfers cholesterol molecules from HDL to other lipoprotein particles, an activity that tends to lower HDL cholesterol levels 2. CETP has been a focus of research since a population of Japanese families was found whose members routinely live for >100 years & carry mutations in the CETP gene 3. A number of small MW CETP inhibitors have been tested in clinical trials & found to greatly increase HDL levels in the blood 4. Whether or not this response correlates with a reduced level of coronary heart disease is currently under investigation The Endocytic Pathway: Moving Membrane and Materials into the Cell Interior Phagocytosis I. Phagocytosis (cell eating) - uptake of relatively large particulate matter (>0.5 µm in dia); extensive in a few cell types specialized for uptake of particulate matter from environment & delivery to lysosomes A. Single-celled heterotrophs (amoebae, ciliates) make their livelihood this way; trap food particles & smaller organisms & enclose them within folds of plasma membrane, engulfing food particles 1. Folds fuse to form vacuole (phagosome) that pinches off inwardly from plasma membrane 2. Phagosome then fuses with lysosome forming phagolysosome, within which material is digested 3. Process is somewhat similar to digestion of cytoplasmic organelle by autophagy B. In most animals, phagocytosis by certain cells is protective mechanism rather than mode of feeding 1. Mammals possess a variety of professional phagocytes (macrophages, neutrophils) – wander through blood & tissues phagocytizing invaders, damaged & dying cells, aging RBCs, debris 2. These materials are recognized & bound by highly selective surface receptors on surface of phagocyte prior to uptake; started by contact of cell with right target 3. Mammalian phagocytosis is markedly enhanced by a number of blood-borne proteins (opsonins) that coat the particle to be ingested 4. Once inside the phagocyte, microorganisms are killed by lysosomal enzymes or oxygen free radicals generated in phagosome lumen II. A proteomic study of macrophage phagosomes revealed the presence of a surprisingly large number of proteins in these seemingly simple membrane-bound vacuoles A. Among the proteins found in phagosome membrane were a number of species characteristic of the ER, including the chaperone calnexin B. It was found that most of the phagosome membrane content of a macrophage is actually derived from ER rather than the plasma membrane 1. It appears that interaction of cell surface with a particle to be engulfed leads to recruitment of ER into the region just beneath the plasma membrane 2. As particle is engulfed, underlying ER fuses with plasma membrane, producing a phagosome membrane composed largely of ER 3. This appears to be one way that phagocytic cells are able to add a large amount of required membrane to their cell surface in a short amount of time III. Phagocytosis is driven by actin-containing microfilament contractile activities underlying cell membrane IV. Not all bacteria ingested by phagocytic cells are destroyed; in fact, some species hijack phagocytic machinery to promote their own survival in body A. Mycobacterium tuberculosis - taken into macrophage cytoplasm by phagocytosis; phagosome doesn't fuse to lysosome; bacterium inhibits fusion that would kill it & multiplies within cell 348 B. Coxiella burnetii (causes Q fever) – its phagosome fuses with lysosome, but neither acid pH nor the lysosomal enzymes can destroy it C. Listeria monocytogenes (causes meningitis) - produces proteins that destroy lysosomal membrane integrity so bacterium can escape into cell cytosol Posttranslational Uptake of Proteins: Peroxisomes I. Protein trafficking in cell is governed by: A. Sorting signals - secreted protein signal peptide or mannose phosphate groups of lysosomal enzymes B. Receptors that recognize these signals & deliver the protein containing them to proper compartment II. Four of cell's major organelles (nucleus, mitochondria, chloroplasts, peroxisomes) import proteins through one or more outer boundary membranes A. Proteins imported by these organelles contain amino acid sequences that serve as addresses recognized by receptors at organelle's outer membrane, as with RER B. Unlike RER, which usually imports proteins cotranslationally, proteins of these organelles are imported posttranslationally (after their complete synthesis on free ribosomes in the cytosol) III. Uptake of proteins into peroxisomes A. Peroxisomes are very simple organelles with only 2 subcompartments in which an imported protein can be placed: boundary membrane & internal matrix B. Proteins destined for peroxisome possess peroxisomal targeting signal: either a PTS for a peroxisomal matrix protein or an mPTS for a peroxisomal membrane protein 1. Several different PTSs, mPTSs & PTS receptors have been identified 2. PTS receptors bind to peroxisome-destined proteins in cytosol & shuttle them to peroxisome membrane prior to import 3. The receptor apparently accompanies peroxisomal protein through membrane into matrix & then recycles back to the cytosol to escort another protein C. Unlike mitochondria & chloroplasts, whose imported proteins must assume an unfolded state, peroxisomes can somehow import peroxisomal matrix proteins in their native, folded conformation 1. This is even true of peroxisomal proteins that consist of several subunits 2. Mechanism responsible remains a matter of speculation Posttranslational Uptake of Proteins: Mitochondria I. Mitochondria have 4 subcompartments into which proteins can be delivered: A. Outer mitochondrial membrane (OMM) B. Inner mitochondrial membrane (IMM) C. Intermembrane space D. Matrix II. Mitochondria synthesize a few of their own integral membrane polypeptides (13 in mammals), but the vast majority of the organelle's proteins (roughly 99%) are encoded by nuclear genome 349 A. These proteins are synthesized in the cytosol & imported posttranslationally B. Proteins of mitochondrial matrix & IMM make up the vast majority of proteins targeted to mitochondria so discussion is restricted to them III. As with peroxisomal proteins & those of other compartments, mitochondrial proteins contain signal sequences that target them to their home-base A. Mitochondrial-matrix proteins have targeting sequence (presequence) found at molecule's N-terminus; it includes a number of positively charged residues that lie on one face of an extended -helix 1. The N-terminal targeting sequence is ultimately removed by a mitochondrial processing protease following import into matrix B. In contrast, most proteins destined for IMM contain several internal sequences that remain as part of the molecule IV. Before a protein can enter a mitochondrion, several events are thought to take place: A. Protein must be presented to a mitochondrion in relatively extended or unfolded state B. Several different molecular chaperones are implicated in preparing polypeptides for mitochondrial uptake, including ones that specifically direct mitochondrial proteins to cytosolic OMM surface 1. Others unfold polypeptides & prevent their aggregation V. OMM contains protein import complex (TOM complex), which includes a receptor that recognizes & binds mitochondrial proteins & a protein-lined channel A. Unfolded polypeptides are translocated through OMM via the protein-lined channel 1. Unlike ER translocon or peroxisome, pore-forming protein of TOM complex is a -barrel protein, like other OMM integral proteins, reflecting its evolution from ancestral bacterium outer membrane 2. Functional consequences - -barrel protein cannot open laterally to allow integral proteins to insert into OMM; OMM proteins must pass into intermembrane space before entering OMM bilayer B. Proteins destined for the IMM or matrix must pass through intermembrane space & engage a second protein-import complex located in the IMM (TIM complex); IMM contains 2 major TIM complexes 1. TIM22 – binds integral proteins of IMM & inserts them into lipid bilayer 2. TIM23 – binds matrix proteins & translocates them completely through IMM into aqueous matrix compartment C. Movement into matrix powered by electric potential across IMM acting on "+"-charged targeting signal 1. If potential is dissipated by addition of drug like DNP, translocation ceases & polypeptide remains trapped within membrane VI. As polypeptide enters aqueous matrix, it interacts with mitochondrial chaperones (e.g., mtHsp70) that mediate entry; 2 mechanisms explain the general chaperone role in protein movement across membranes: A. According to one view, chaperones act as force-generating motors that use energy from ATP hydrolysis to "pull" the unfolded polypeptide through the translocation pore B. Alternate view - chaperones aid in polypeptide diffusion across membrane; this is a random process in which a molecule can move in any available direction 1. Protein protrudes into matrix through translocation pore, a chaperone residing on the inner surface of the membrane grabs it & keeps it from diffusing back out through the pore and into the cytosol 2. The chaperone, however, did not block the protein's diffusion further into the matrix 3. As polypeptide diffuses further into matrix, it binds repeatedly to the chaperone & at each stage prevented from diffusing backward out of matrix; mechanism called biased diffusion 4. The chaperone is said to be acting as Brownian ratchet (the term Brownian implies random diffusion; a ratchet is a tool that allows movement in only one direction) 350 C. Recent evidence suggests that both of the above mechanisms of chaperone action are probably used & act cooperatively Posttranslational Uptake of Proteins: Chloroplasts I. Chloroplasts have 6 subcompartments into which proteins can be delivered: A. Inner (1) & outer (2) envelope membrane & intervening intermembrane space (3) B. Stroma (4), thylakoid membrane (5), thylakoid lumen (6) II. Chloroplast & mitochondrial import mechanisms exhibit many similarities, although their translocation machinery have evolved independently; as in mitochondria: A. Vast majority of chloroplast proteins are imported from cytosol B. The outer & inner envelope membranes contain distinct translocation complexes (Toc & Tic complexes, respectively) that work together during import C. Chaperones aid in the unfolding of the polypeptides in the cytosol & folding of the proteins in the chloroplast, and D. Proteins destined for the chloroplast are synthesized with a removable N-terminal sequence (termed the transit peptide) III. Transit peptide does more than simply target a polypeptide to a chloroplast; it provides an address that localizes the polypeptide to one of several possible subcompartments within the organelle A. All proteins translocated through the chloroplast envelope contain a stroma-targeting domain as part of their transit peptide; this guarantees that the polypeptide will enter the stroma 1. Once in the stroma, the stroma-targeting domain is removed by a processing peptidase located in that compartment B. Those polypeptides that belong in a thylakoid membrane or a thylakoid lumen bear an additional segment in their transit peptide (thylakoid transfer domain), that dictates entry into the thylakoids C. Several distinct pathways have been identified by which proteins are either inserted into the thylakoid membrane or translocated into the thylakoid lumen 1. These pathways have striking similarities to transport systems in bacterial cells, the presumed ancestors of chloroplasts 2. Many of the proteins residing within thylakoid membrane are encoded by chloroplast genes & synthesized on membrane-bound ribosomes of the chloroplast THE HUMAN PERSPECTIVE: DISORDERS RESULTING FROM DEFECTS IN LYSOSOMAL FUNCTION I. First discovery of mechanism for targeting proteins to particular organelles was discovery that mannose 6phosphate residues in lysosomal enzymes act as an address for delivery of these proteins to lysosomes A. Discovery of lysosome address was made in studies of patients with a rare & fatal inherited condition (Icell disease) B. Many cells contain bloated lysosomes that are bloated with undegraded materials; this happens because of the absence of hydrolytic enzymes C. When fibroblasts from these patients were studied in culture, it was found that lysosomal enzymes are synthesized at normal levels but they are secreted into medium & not targeted to lysosomes D. Further analysis showed that the secreted enzymes lacked mannose phosphate residues present on the corresponding enzymes of cells from normal individuals E. The I-cell defect was soon traced to a deficiency of an enzyme (N-acetylglucosamine phosphotransferase) required for mannose phosphorylation 351 II. H. G. Hers (Univ. of Louvain in Belgium, 1965) – explained how absence of seemingly unimportant lysosomal enzyme (-glucosidase) leads to development of fatal inherited condition (Pompe disease) A. He suggested that in absence of -glucosidase, undigested glycogen accumulated in lysosomes, causing swelling of organelles & irreversible damage to cells & tissues B. Diseases of this type, characterized by deficiency of a single lysosomal enzyme & the corresponding accumulation of undegraded substrate, are called lysosomal storage disorders 1. >40 of these have been described, affecting ~8,000 infants; their symptoms can range from very severe to barely detectable, depending primarily on the degree of enzyme dysfunction 2. Several diseases have also been traced to mutations in lysosomal membrane proteins that impair the transport of substances to the cytosol III. Among the best-studied lysosomal storage disorders is Tay-Sachs disease, which results from a deficiency of the enzyme -N-hexosaminidase A, an enzyme that degrades the ganglioside GM2 A. GM2 is a major component of brain cell membranes 1. In the absence of hydrolytic enzyme, ganglioside accumulates in brain cell cytoplasm causing a dysfunction 2. In its severe form, which strikes during infancy, the disease is characterized by progressive mental & motor retardation, as well as skeletal, cardiac, & respiratory abnormalities B. The disease is very rare in the general population but reaches an incidence up to 1 in 3600 newborns among Jews of eastern European ancestry 1. Disease incidence has dropped dramatically in this ethnic population recently as a result of the carrier identification, genetic counseling of parents at risk & prenatal diagnosis by amniocentesis 2. All of the lysosomal storage disorders can be diagnosed prenatally IV. Recently, prospects for treatment of lysosomal storage disorders have improved with demonstration that the symptoms of Gaucher's disease can be alleviated by enzyme replacement therapy A. Gaucher's disease is a deficiency of the lysosomal enzyme glucocerebrosidase B. Infants with Gaucher's disease accumulate large quantities of glucocerebroside lipids in macrophage lysosomes, causing liver & spleen enlargement & anemia C. Initial attempts to correct the disease by infusing a solution of normal human enzyme into bloodstream were unsuccessful since enzyme was taken up by liver cells (not seriously affected by the deficiency) D. To target macrophages, enzyme was purified from human placental tissue & treated with 3 different glycosidases to remove terminal sugars on the enzyme's oligosaccharide chains 1. This exposed the underlying mannose residues 2. After infusion into bloodstream, this modified enzyme (marketed under name Ceredase) or the newer recombinant enzyme Cerezyme is recognized by mannose receptors on macrophage surface 3. They are then rapidly taken up by receptor-mediated endocytosis 4. Since lysosomes are natural target site of materials brought into macrophage by endocytosis, the enzymes are efficiently delivered to the precise sites in the cell where the deficiency is manifested 5. Thousands have been successfully treated in this way E. Clinical trials of enzyme replacement therapy for treatment of several other lysosomal storage disorders are also showing promising results 1. Unfortunately, many of these diseases affect the central nervous system, which is unable to take up circulating enzymes because of the blood-brain barrier F. Alternate approach has shown some promise in preclinical trials; called substrate reduction therapy, in it small MW drugs are administered to inhibit synthesis of substances that accumulate in the disease G. Finally, although it leads to considerable risk to the patient, bone marrow (or cord blood) transplantation has proven relatively successful in treating some of these diseases 1. It is thought that the foreign transplanted cells, which contain normal copies of the gene in question, secrete a limited amount of the normal lysosomal enzyme 352 2. Some of these enzyme molecules are then taken up by the patient's own cells, which lessens the impact of the enzyme deficiency EXPERIMENTAL PATHWAYS: RECEPTOR-MEDIATED ENDOCYTOSIS I. Embryonic development begins when small sperm & much larger egg (develops from oocyte, which accumulates yolk made elsewhere in female's body) fuse – how do high-MW yolk proteins enter oocyte? A. Thomas Roth & Keith Porter (Harvard, 1964) – reported on mechanism by which yolk proteins might be taken into mosquito oocytes 1. During stages of rapid oocyte growth, there was dramatic increase in the number of pitlike depressions seen on oocyte surface 2. The pits, formed by invagination of oocyte plasma membrane, were covered on their inner surface by a bristly coat 3. Roth & Porter postulated that yolk proteins were specifically adsorbed onto the outer surface of the membranes of the coated pits, which would then invaginate as coated vesicles 4. The coated vesicles would lose their bristly coat & fuse with one another to produce the large, membrane-bound yolk bodies characteristic of the mature oocyte B. Toku Kanaseki & Ken Kadora (Univ. of Osaka, 1969) – provided insight into structure of coated vesicles; did EM examination of crude vesicle fraction isolated from guinea pig brains 1. Coated vesicles were covered by a polygonal basketwork 2. Suggested that coatings were an apparatus to control the infolding of plasma membrane during vesicle formation II. Barbara Pearse (Medical Research Council, Cambridge, England, 1975) – first studies of biochemical nature of the vesicle coat A. Developed procedure in which membrane vesicles from pig brains were centrifuged through a succession of sucrose density gradients until a purified fraction of coated vesicles was obtained B. Protein from coated vesicles was solubilized & fractionated by SDS-PAGE; the coat cotained one predominant protein species with a molecular mass of ~180,000 daltons; called the protein clathrin C. Found the same protein (based on molecular mass & peptide mapping) in preparations of coated vesicles that were isolated from several different types of cells obtained from >1 animal species III. Michael Brown & Joseph Goldstein (Univ. of Texas Med. Sch. Dallas TX; 1973) – independent line of research; interested in inherited condition familial hypercholesterolemia (FH) A. People homozygous for defective gene (FH allele) had profoundly elevated serum cholesterol levels (800 mg/dl vs. 200 mg/dl for a normal person) 1. They invariably developed severely blocked (atherosclerotic) arteries & usually died from heart attack before the age of 20 2. At that time, very little was known about the fundamental physiologic or biochemical defects in the disorder B. Began studies by examining cholesterol metabolism in cultured fibroblasts derived from the skin of normal & FH-afflicted individuals 1. Found that rate-controlling enzyme in cholesterol biosynthesis, HMG CoA reductase, could be inhibited in normal fibroblasts by cholesterol-containing lipoproteins (like LDL) placed in medium 2. LDL addition to culture medium in which normal fibroblasts were growing led to decreased level of HMG CoA reductase activity & corresponding decrease in cholesterol synthesis by fibroblasts 3. When HMG CoA reductase levels were measured in FH-derived fibroblasts, they were found to be 40 – 60 times that of normal fibroblasts 4. Also, enzyme activity in FH fibroblasts was totally unaffected by presence of LDL in medium C. How could lipoprotein in medium affect activity of enzyme in cytoplasm of cultured cells? – Brown & Goldstein initiated studies on the interaction between the cells & the lipoproteins 353 1. Added radioactively labeled LDL to culture dishes containing a single layer of fibroblasts derived from either FH-afflicted or normal human subjects 2. The normal fibroblasts bound the labeled LDL molecules with high affinity & specificity, but the mutant cells showed virtually no ability to bind these lipoprotein molecules 3. This indicated that normal cells have a highly specific receptor for LDL & that the receptor was defective or missing in cells from patients with FH D. Brown & Goldstein teamed up with Richard Anderson who was studying cell structure with the EM 1. They incubated fibroblasts from normal & FH subjects with LDL that had been covalently linked to the iron-containing protein ferritin, which because of iron content can scatter an electron beam 2. Thus it can be visualized in EM 3. When normal fibroblasts were incubated with LDL-ferritin at 4°C, a temperature at which ligands can bind to cell surface but cannot be internalized, LDL-ferritin particles were bound to cell surface 4. The LDL particles were not randomly scattered over the cell surface, but were localized to short segments of plasma membrane where the membrane was indented & coated by a fuzzy material 5. These segments of membrane were similar to the coated pits described by Roth & Porter & had since been seen in a variety of cell types 6. Cells from FH patients had a similar number of coated pits on surface but no LDL-ferritin was bound to these mutant cells 7. Concluded that mutant FH allele encoded a receptor that was unable to bind LDL 8. Subsequent EM studies on LDL-ferritin internaliztion revealed the endocytic pathway by which these lipoprotein particles were internalized E. They postulated that rapid internalization of receptor-bound LDL is strictly dependent on localization of LDL receptors in coated pits 1. If LDL receptor failed to be localized within coated pit, it would not be able to deliver its bound ligand to cellular lysosomes & thus would not be able to affect cholesterol biosynthesis within cell IV. An LDL receptor with a different kind of mutation was soon found A. This defect (known as J. D. mutation after the patient in which it occurred) bound normal amounts of radioactively labeled LDL, yet receptor-bound LDL failed to be internalized 1. Thus, it was not delivered to cytoplasmic lysosomes for processing B. Anderson et al. postulated that LDL receptor was transmembrane protein that normally was localized in coated pits, because its cytoplasmic domain was specifically bound by coated pit component 1. The component was thought to possibly be clathrin but was later identified as as a likely subunit of an AP adaptor 2. Because of a defect in its cytoplasmic domain, the J. D. mutant receptor was unable to localize in a cell's coated pits 3. People with this mutation exhibit same phenotype as patients whose receptors cannot bind LDL C. Later studies showed that normal LDL receptor is 839 amino acid transmembrane glycoprotein, with 50 amino acids at C-terminal end of protein extending inward from membrane as cytoplasmic domain 1. The protein contained a single amino acid substitution; a tyrosine residue normally located at position 807 was replaced by a cysteine 2. This single change in amino acid sequence obliterated protein's ability to concentrate in coated pits D. Attention turned to the amino acid sequences of the cytoplasmic tails of other receptors that get localized to coated pits – were there any common internalization signals? – 2 such signals turned up 1. Both contained tyrosine (Y in single letter nomenclature): a less common NPXY signal (as in the LDL receptor) & YXX signal (as in the transferrin receptor) 2. In the YXX signal, X can be any amino acid & is an amino acid with a bulky, hydrophobic side chain 3. The YXX sequence of the receptor binds to the µ subunit of the AP2 adaptors; X-ray crystallography has revealed the nature of interaction between adaptor & internalization signal 4. Meanwhile, the AP2 adaptor complex binds to the clathrin coat by means of its subunit 354 5. As a result of these various intermolecular contacts, the adaptor complex & receptor are trapped in coated pits prior to endocytosis V. Lines of investigation about clathrin-mediated endocytosis followed over the past decade A. ≥2 components of endocytic machinery have been labeled with different fluorescent markers & their movements were followed over time within a living cell 1. Dynamin, AP2 adaptors or various types of cargo receptors have been seen to bind to clathrin-coated pits & get enveloped in clathrin-coated vesicle, which buds off into cytoplasm & disappears B. Engineer cultured mammalian epithelial cells to express a clathrin light chain that is fused to a variant of the green fluorescent protein 1. When observed by fluorescence microscopy, see green patches on cell surface, which for the most part represent clathrin-coated pits situated at the cell surface 2. Red-staining spots on slide are individual fluorescently labeled LDL particles that were added to the medium in which the cells were growing 3. Once LDL particle has been bound to coated pit LDL receptor, the overlap of the two fluorescent dyes produces a yellow-orange spot 4. Later see uncoated vesicle with containing red fluorescent LDL particles moving into the adjacent cytoplasm QUESTÕES 1. O mRNA de uma protein secretora bem conhecida foi isolado e colocado num tubo de ensaio, juntamente com outras substâncias necessárias à síntese proteica in vitro. A sequência da proteína produzida in vitro era diferente da sequência da proteína secretora purificada. Qual a possível explicação para este facto? 2. Submeteram-se células em crescimento a um pulso breve com radioactividade, tornado radioactivos os fosfolípidos. Algum tempo depois, observou-se radioactividade primeiramente sobre o retículo endoplasmático. Posteriormente, aparece na membrana celular, logo após ter sido observada sobre o complexo de Golgi e em vesículas citoplasmáticas. A que conclusões apontam os dados apresentados? 3. Uma molécula chamada aequorina produz fluorescência quando se liga a iões Ca2+. Se estiver a observar uma célula na qual se injectou aequorina e se essa célula segregar determinadas proteínas, o que se observa imediatamente antes da secreção dessas proteínas? 4. Se uma ameba for tratada com citocalasina B, um inibidor da polimerização da actina, o processo de fagocitose pára. Qual razão se isso acontecer? 5. Uma mulher deu à luz um bebé incapaz de adquirir adequadamente os anticorpos provenientes do leite materno. Posteriormente, determinou-se que ele produzia receptores para os anticorpos e que estes se ligavam a esses receptores. Indique uma possível razão para a incapacidade do bebé adquirir os anticorpos maternos! 6. Qual é a causa da silicose e da asbestose? 7. Porque razão as enzimas lisossomais causam artrite reumatóide quando são libertadas para o espaço extracelular? 355 8. Explique porque é que os lisossomas ficam inchados nos pacientes que sofrem da doença das células I! 9. Qual é a doença caracterizada pela ausência ou malformação da enzima α-glucosidase? Qual o papel fisiológico da enzima α-glucosidase? 10. Na figura 8.2b, o que aconteceria à taxa com que a via constitutiva ocorre se a via secretora regulada recebesse estímulos que reduzissem a taxa de secressão? 11. Qual é a função das cisternas achatadas e empilhadas do retículo endoplasmático rugoso, observadas na figura 8.9a-d? 12. Relativamente à figura 8.11a: a) Quais os organelos localizados na parte basal da célula? b) Quais os organelos localizados na parte apical da célula? c) Onde se encontra o complex de Golgi? 13. Na figura 8.13, o que impede a cadeia polipeptídica de uma proteína membranar integral que está a ser formada de passar completamente através do translocão? 14. A figura 8.14 representa a adição assimétrica de grupos de oligossacarídeos a proteínas. Se uma dada proteína integral tiver alguns açúcares no lado citoplasmático da membrana plasmática, em qual das extremidades dessa proteína terão sido adicionados os açúcares? 15. De acordo com a figura 8.16, em qual das superfícies do Retículo Endoplasmático são adicionados os primeiros açúcares ao fosfato de dolicol e de que compartimento são obtidos os últimos açúcares a serem adicionados? 16. De acordo com a figura 8.27, o que aconteceria se fosse adicionado GTP em excesso relativamento ao seu análogo GTPγS? 17. A figura 8.29b mostra a sequência de eventos envolvidos no direccionamento de enzimas lisossomais para os lisossomas. O que aconteceria se fosse bloqueada a fosforilação de resíduos de manose das enzimas lisossomais? 18. De acordo com a figura 8.29, se uma pessoa tivesse uma alteração genética que resultasse em receptores de enzimas lisossomais deficientes, quais seriam os efeitos fisiológicos desta alteração? Esta pessoa teria um problema médico? 19. De que modo se relacionam os níveis de LDL com os ataques cardíacos? 356