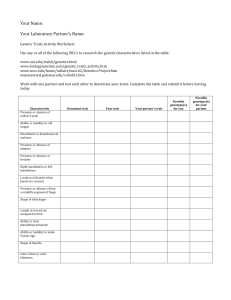
The genetics of fish behavior
advertisement

1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 The genetics of fish behavior Alison M. Bell University of Illinois, Urbana-Champaign alisonmb@life.uiuc.edu Introduction This is an exciting time to study the genetics of behavior (Boake et al., 2002; Fitzpatrick et al., 2005; Robinson et al., 2005). Until recently, the powerful tools needed to understand genetic influences on behavior were available for just a few model organisms that were not widely studied by behavioral ecologists (Fitzpatrick et al., 2005; Robinson et al., 2005). Moreover, the behaviors that could be studied were often simple and were measured in laboratory environments rather than in the field. From a genetic perspective, behavior was considered to be too subjective to measure, too susceptible to environmental influences, too plastic and not repeatable. Fear of accusations of genetic determinism and carryovers from the troubling political implications of eugenics and sociobiology might also have contributed to the underrepresentation of behavior among traits studied from a genetic perspective (Lewontin et al., 1984). However, behavioral traits are not alone in their sensitivity to environmental influence: nonbehavioral traits such as morphological traits, which we know are amenable to genetic dissection, can be plastic and responsive to the environment as well (e.g. trophic morphology: (Adams et al., 2003; Bell, 2005; Chapman et al., 2000), body size: (Losos et al., 2000; Wikelski and Thom, 2000)). At the same time, there is growing appreciation that behavior might not be as plastic as we had assumed (Sih et al., 2004; Sih et al., 2004; West-Eberhard, 2003). Moreover, we know that behavioral traits respond to both natural and sexual selection. Behaviors can be heritable, with heritability estimates for behaviors broadly comparable to other kinds of traits (reviewed in (Meffert et al., 2002; Roff and Mousseau, 1987; Stirling et al., 2002)), and they show adaptive, heritable geographic variation. Fishes show particularly good examples of geographic variation in behavior, especially guppies (Poecilia reticulata, Endler, 1995), sticklebacks (Gasterosteus aculeatus, Bell and Foster, 1994), and Arctic charr (Salvelinus alpinus, summarized in (Foster and Endler, 1999)). Finally, increasing evidence that behavior can be studied from a phylogenetic perspective (Brooks and McLennan, 1991), and an appreciation of behavior’s role in evolutionary processes such as speciation and reproductive isolation (Boughman, 2002) have all contributed to interest in bridging the gap between genetics and behavior. This chapter is written by and for behavioral ecologists working primarily from the behavior ‘down’ (or ‘forward’, if you’re a geneticist) to genes. That is, I start with ecologically-relevant natural variation in behavior and discuss approaches for studying the genes underlying that variation. This strategy is alternative to approaches which start with artificially-induced mutations and ask their consequences for behavior. Essentially, I will try to make a case for why scientists interested in behavior might want to include a genetic component in their research program and to briefly describe some of the tools available to them. 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 As the most diverse vertebrate taxon, fishes provide fascinating ecologically relevant behavioral variation to be analyzed from a genetic perspective. For example, fishes exhibit a greater diversity of mating systems than any other vertebrate taxon (Helfman et al., 2000). Fish are especially suited for genetic studies because many species have external fertilization, which makes it relatively easy to create specific crosses. An additional advantage of studying fishes is that several fish species from diverse groups have had their genome sequenced. At the moment, assembled genomes of the following fish species are available: Fugu (Fugu rubripes, 393MB), zebrafish (Danio rerio 1,688MB), tetraodon (Tetraodon nigroviridis, 402MB) and sticklebacks (Gasterosteus aculeatus, 675MB). Why might a behavioral ecologist study the genetics of behavior? In a classic paper, Alan Grafen proposed that with a few simplifying assumptions, behavioral ecology might be able to ignore genetics (Grafen, 1984). He argued that the aim of behavioral ecology is to uncover the selective forces that shape characters, and that our method will work almost regardless of which genetic system underlies the character. He proposed ‘the phenotypic gambit’, which was ‘to examine the evolutionary basis of a character as if the very simplest genetic system controlled it’ (Grafen, 1984). Here, I take seriously Grafen’s challenge to find cases where an understanding of the genetic mechanisms makes a difference. I highlight cases where different genetic mechanisms occur and change the evolutionary dynamics. I make the case that we often need to understand at least a little about the genetic mechanisms underlying behaviors if we are really interested in predicting the response to selection, inferring evolutionary history and understanding how animals cope with a nonequilibrial world. However, for some questions, the phenotypic gambit might be justified. That is, if our interest is purely in the current utility of a trait rather than the evolutionary history or fate of it, the genetic mechanisms underlying the trait might not be informative. For example, if we want to know whether females prefer males with particular ornaments, then we do not need to know the genetics underlying female preference or the male ornament. But there are several reasons why a behavioral ecologist might be interested in the genetics of behavior. We cannot understand the selective forces that have produced adaptive variation without studying genetics in some form. In fact, many of our models either implicitly or explicitly include a genetic component to the behavior of interest (e.g., Trivers, 1971). Optimality models, for example, the simplest models in behavioral ecology, assume the presence of heritable variation for selection to act upon (Orzack and Sober, 1994). Therefore, if we want to know if the behavior has responded to natural or sexual selection in the past, and if it has the potential to evolve, we need to exclude the possibility that variation is not entirely environmentally-induced; we need to know if the trait is heritable. If we are interested in obtaining more detailed information about the rate and direction of past and future evolution, then knowing more about the genetic architecture underlying a trait becomes important. In what follows, I describe what we can learn about the past and future evolution of a particular behavior by elucidating its genetic architecture, including the number of loci, the number of alleles at a given locus, the 2 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 distribution of effect sizes of the genes, whether the trait is genetically correlated with other traits, the relationship between loci (epistasis) and the relationship between alleles at a particular locus (dominance). Understanding genetic architecture is important for behavioral ecologists interested in tracing the evolutionary history of a particular behavior ( e.g., Ruber et al., 2004), as well as for those trying to predict how animals will respond to a changing environment, including anthropogenic-induced change (Schlaepfer et al., 2002). First, the number of genes affecting a behavior and the distribution of their effect sizes determines how the behavior can respond to future environmental change. For example, if a single gene of major effect is responsible for variation in the behavior, the behavior can quickly respond to selection. While traits that are affected by just a few genes might respond faster to selection, it has also been argued that polygenic traits might be more ‘evolvable’ because many genes of small effect effectively increases the opportunities for a beneficial mutation to arise. This additional pool can act as a reservoir for genetic variation, possibly allowing a population to respond to novel selection pressures (Houle et al., 1996). Second, we frequently want to know whether there is additive genetic variation (Va, reflected in the number of alleles at a given locus) for a trait because this can tell us about the past selective regime and the potential for future evolution. Theoretical models predict that natural selection will erode additive genetic variation, therefore small values of Va (or CVa , Houle 1992)) can indicate that the trait has been subject to selection in the past. This reasoning should be treated with some caution, however, because the data actually suggest that there is still substantial genetic variation for fitness-related traits (Merila and Sheldon 1999), which presumably have been subject to strong directional selection in the past. A possible resolution to this paradox is that fitness-related traits are probably affected by many loci, thereby providing a bigger target for mutation to maintain genetic variation (Houle et al., 1996; Merila and Sheldon 1999). As far as the fate of traits under selection goes, a trait will not respond to natural selection unless there is additive genetic variation for it, so knowing Va can tell us whether the trait can respond to selection in the future. For example, global warming is causing disruption to animals that rely on seasonal cues for timing reproduction and migration (Both and Visser, 2001; Visser and al, 1998). If populations do not harbor sufficient genetic variation for resetting the timing of these critical behavioral decisions, then the viability of these populations might be threatened. A third important component of the genetic architecture is how traits are genetically related to each other. A significant genetic correlation between two traits might indicate that the traits have been subject to correlational selection in the past. Correlational selection occurs when a trait’s fitness effect depends on its interaction with another trait (Lande and Arnold, 1983). Alternatively, a genetic correlation might be the result of a fundamental constraint that links two traits together, i.e. pleiotropy. Genetic correlations can also tell us about future responses to selection of a behavior of interest (for an excellent review, see Arnold, 1994)). The response to selection on a particular trait depends on more than just the amount of genetic variation present for it; it also depends on the sign and magnitude of genetic covariance between traits as well as on the force of selection acting on correlated traits (Lande and Arnold, 1983). In some cases, the 3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 response to selection on a given trait can actually be in the opposite direction that we would predict if we did not consider genetic correlations (Grant and Grant, 1995). There is another reason why researchers studying animal behavior might be interested in genetic correlations. That is because genetic correlations hold a central place in sexual selection theory, arguably the most popular and controversial topic in animal behavior. Different theories have been proposed to explain the evolution of exaggerated male ornaments used to attract mates. Fisherian models of sexual selection explain ‘runaway’ evolution of male ornaments by a genetic correlation between ornament and female preference for that ornament. In other words, brothers which have the exaggerated ornament have sisters with a strong preference for that ornament. It has proven difficult to empirically test this model (Qvarnström et al., 2006), but some of the best evidence showing support for the Fisherian process comes from studies of fishes. For example, female guppies prefer to mate with males with particular coloration patterns (especially increased orange area), and evidence from both population comparisons (Houde and Endler, 1990) and selection experiments (Houde, 1994; Brooks and Couldridge, 1999) support the hypothesis that there is a significant genetic correlation between female preference and different male ornaments in this species. This correlation has also been found in threespined sticklebacks (Bakker, 1993). Fourth, nonadditive effects such dominance and epistasis, which are common for behavioral traits (Meffert et al., 2002), also affect our ability to infer past selective regimes and to predict evolutionary potential. In a recent review, Merila and Sheldon (1999) reported that nonadditive genetic effects (and environmental effects) are the most important genetic determinants of fitness in nature. Dominance refers to the relationship between alleles at a given locus; a dominance relationship implies that one allele is dominant to its partner. At the opposite end of the spectrum, if two alleles contribute equally, then the relationship between alleles is said to be additive. Directional selection can favor the evolution of directional dominance for the allele that corresponds to the favored phenotype (Broadhurst and Jinks, 1979). Therefore, a dominance relationship between alleles at a given locus might indicate that the gene has been subject to directional selection in the past. Similarly, because a dominant allele will reach fixation faster than a recessive allele, the dominance relationship among alleles influences how quickly the trait can evolve (Falconer, 1989). Epistasis refers to interactions between loci affecting a trait. Selection can favor particular interactions between loci, resulting in coadapted epistatic gene complexes within populations. Epistasis is important for evolution because outbreeding depression can occur when populations that differ in their coadapted gene complexes are crossed. The breakdown of coadapted gene complexes can result in reproductive isolation between populations with different genetic backgrounds (Meffert et al., 2002). Therefore, epistasis could be a mechanism for maintaining postzygotic reproductive isolation between populations according to the ‘Dobzhansky-Muller model’ (Dobzhansky, 1937). For some people studying the genetics of complex traits, the real trophy is to find genes. If genes associated with adaptation can be identified, that can allow us to trace the evolutionary history of the gene through time. When did the gene originate? What are the functions of the gene in phylogenetically-distant relatives? Are similar behaviors related to the same gene in distant relatives? Is the same gene used over and over again to accomplish similar functions? Does this occur via de novo mutation in particular ‘hot 4 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 spots’ of the genome, or is there standing genetic variation for it? We are beginning to get a glimmer of answers to these perennial questions for nonbehavioral traits (Colosimo et al., 2005; Hoekstra et al., 2004) and it is only a question of time until we can answer those kinds of questions about behavioral traits as well (Fitzpatrick and Sokolowski, 2004). Finally, we might want to find genes so that we can manipulate them. This is desirable, of course, for clinical applications, including psychopharmacology (Stahl and Muntner, 2000) but it could also be a powerful experimental tool (a la ‘phenotypic engineeering’ (Ketterson et al., 1996; Sinervo and Huey, 1990)) for the behavioral ecologists’ toolbox. Approaches for studying the genetics of behavior and who can use them In this section, I describe in order of increasing complexity how a researcher might approach the genetics of a behavior of interest. For each method, I describe its aim, the general approach, its strength and weaknesses and the kinds of traits and animals for which it is most suited. ‘Low tech’ approaches The first step in approaching the genetics of a behavioral trait is to determine if the behavior is repeatable, which involves measuring the same behavior on the same individual several times (Lessels and Boag, 1987). Repeatability is the proportion of phenotypic variance attributable to the individual, which could be caused by additive genetic variance or environmental variance with long-lasting effects. Evidence that a behavior is repeatable indicates that it might have a heritable component and is amenable for further genetic dissection. The repeatability of a trait sets an ‘upper bound’ to heritability (Boake, 1994). However, it is worth considering that nongenetic effects can also produce stable behavior; repeated reinforcement including learning can also lead to stability (Stamps, 2003). Another indication that there might be a heritable component to the behavior is if it is consistent across contexts; in this case, the stability occurs via a correlation between individual behaviors in different contexts, or a behavioral syndrome, which are discussed further, below (Sih et al., 2004; Sih et al., 2004). Another relatively low-tech approach to the genetics of a behavior is to ask if it differs across populations that occur in different kinds of environments (Foster and Endler, 1999). If so, then population differences in the behavior could reflect an evolved response to differing selective pressures. Alternatively, the differences could reflect environmentally-induced changes within individuals. A way to disentangle these two hypotheses is to rear animals from the different populations in a ‘common garden’, or in the same environment. If population differences in the behavior are preserved under common environmental conditions, then the difference between populations might be genetic in origin. A common garden experiment represents the first step toward approaching the genetics of complex traits such as behavior, but there are at least two important factors to consider before concluding that population differences are genetic in origin. The first is that F1s reared in a common garden could still experience parental 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 effects from their wild-caught parents. To control for such environmental effects, it is preferable to rear the offspring of lab-born individuals and to compare the phenotype of the F2s. Second, GxE interactions should also be considered when populations do not differ in a common environment. For example, individuals from both populations might converge in their response to the common garden environment, but the extent of response might have a genetic component. A reciprocal transplant experiment might reveal genetic differences that are not apparent in a common environment. An advantage to using population differences as a clue to which behaviors are likely to be heritable is that traits that differ across populations in different environments are probably linked to fitness. Several common garden studies on fishes have shown that population differences in behavior are often preserved in a common garden (Grether et al., 2001; Lahti et al., 2001; Pakkasmaa and Piironen, 2001; Palm and Ryman, 1999). For example, Lahti et al. 2001 found evidence for a genetic basis to aggressive behavior in brown trout by comparing different types of populations. The authors reared fish from 10 populations in a common environment. The populations can be grouped into the following types: resident (nonmigratory), sea-run (migratory) or lake-run (migratory), so there were replicate populations for each type. Contrary to the expectation that resident trout are more aggressive than migratory forms, they found that sea-run populations were consistently more overtly aggressive toward opponents than the other types of populations. There is a rich literature comparing domesticated and wild populations of salmonids, which has revealed genetic influences on several types of behavior (reviewed in (Huntingford, 2004)). When reared in a common garden, hatchery fish show reduced antipredator responses (Alvarez and Nicieza, 2003; Malavasi et al., 2004; Petersson and Järvi, 2006), are more bold toward a novel object (Sundström et al., 2004) and are frequently more aggressive (Lepage et al., 2000). However, wild Atlantic salmon (Salmo salar) became dominant over hatchery salmon if they were given an opportunity to establish residence (Metcalfe et al., 2003), suggesting that the expression of genetic differences between hatchery and wild salmon depends on the environmental context. Quantitative genetic approaches There is a long history of examining the genetic basis of continuously distributed traits using quantitative genetic techniques, which are based on the phenotypic resemblance among relatives due to shared genes. Unlike some of the reverse methods described below, quantitative genetic techniques measure phenotypes, not genes, and rely on the resemblance among relatives to infer genetic effects. Quantitative genetic approaches assume that many genes of small effect influence the phenotype, thereby producing a continuous distribution (however, recent QTL studies have called this assumption into question). By measuring the phenotype on individuals of known relatedness, we can partition the phenotypic variation into environmental, genetic and gene x environment variance components (Falconer, 1989). The simplest quantitative genetic approach is to ask whether the trait of interest runs in families, which would suggest that there might be a genetic component to it. However, families often share a common environment so cross fostering experiments are useful here (Boake, 1994; Boake et al., 2002). Like repeatability, a demonstration of full- 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 sib resemblance sets an upper limit to the heritability. More complicated breeding designs involve estimating the resemblance of parents and offspring using regression, or generating full and half-sib families to disentangle parental effects from genetic effects using ANOVA-based approaches, which are thoroughly described elsewhere (Falconer, 1989; Lynch and Walsh, 1998). These approaches estimate heritabilities, genetic correlations, parental effects and non-additive genetic variance. The most powerful quantitative genetic experiments estimate the G matrix. The G matrix refers to the multivariate matrix of genetic variances and covariances between several different traits. Although laborious to quantify, obtaining a G matrix allows us to predict the consequences of selection on any given trait, and, with a few assumptions about the stability of the G matrix through time, allows us to retrospectively analyze selection in the past (Jones et al., 2003; Lande, 1979). In addition to satisfying the assumption of stability required for retrospective selection analysis, it is also desirable to compare G matrices to determine whether genetic constraints are limiting. If genetic correlations can be uncoupled, then the relationship between traits might respond to selection, and the G matrix will, itself, evolve. Alternatively, genetic correlations might limit the number of different configuration of traits that are possible, which would be reflected in an invariant G matrix through time. Fortunately, there are several different techniques available to compare the structure of G matrices (Steppan, 2002) but there is debate about the best method. A relatively new powerful technique known as the animal model allows us to estimate genetic variances and covariances without performing breeding experiments in the lab (Kruuk, 2003). Therefore this method is particularly useful for field studies. The animal model is powerful because it takes advantage of all types of resemblance between relatives to partition variance components; it uses all the available information about relatedness from a pedigree. Moreover, this method is suitable for unbalanced datasets, and can accommodate missing values. Another approach for estimating genetic variances and covariances is to perform an artificial selection experiment, which involves selective breeding of individuals. Selection experiments have shown that many behavioral traits can respond to selection (Boake, 1994). Artificial selection experiments are only suitable for organisms that can be kept in the lab, and which ideally have a short generation time. Selection experiments should only be undertaken when different selected lines can be replicated and when sample sizes are sufficient to control for drift and inbreeding. The pitfalls and perils of selection experiments are reviewed in (Fuller et al., 2005). Artificial selection experiments can be particularly insightful when they vary the environmental context in which selection occurs. For example, in a very interesting series of selection experiments on medaka (Oryzias latipes, Ruzzante and Doyle ( 1991, 1993) showed that the correlated response of aggressiveness to selection for fast growth depended on the ecological context in which the selection took place. In these experiments, Ruzzante and Doyle selected for fast growth under two different conditions: when food was clumped and could be defended, and when food was dispersed. The same limited amount of food was added to each treatment. Selection for fast growth produced a correlated response to selection on aggressiveness, but only when food was defensible. Interestingly, levels of aggressiveness decreased in the fast-growth lines, which they interpret as reflecting indirect selection for ‘social tolerance’. 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 One of the strengths of a selection experiment is that it can tell us whether there are packages of traits that are linked together and respond to selection in concert. For example, selection for increased and decreased stress responsiveness, as measured by the change in circulating concentrations of cortisol in response to handling stress, produced several correlated effects on physiological and behavioral measures in rainbow trout (Oncorhynchus mykiss, Pottinger and Carrick, 1999). Relative to trout that did not release very much cortisol in response to handling stress, ‘high responding’ trout were more aggressive in a new environment (Schjolden et al., 2005), were more active in the presence of an intruder and took longer to acclimate to a new environment (Øverli et al., 2002). Moreover, these behavioral differences were also accompanied by changes in the brain monoaminergic systems (Øverli et al., 2001). These results suggest that an entire suite of physiological and behavioral changes accompanied modifications to stress responsiveness. Interestingly, the packages of physiological and behavioral traits that changed together are analogous to different ‘coping styles’ that have been identified in other vertebrates (Koolhaas et al., 1999). Candidate gene approaches Over the past few decades, there has been increasing evidence that the molecular functions of many genes are highly conserved across species. For example, studies on transgenics have revealed that genes from one species accomplish similar functions in distantly-related species (Jaenisch, 1988; Manzanares et al., 2000). The incredible conservation of gene function in living things allows us to apply genetic information about other species to the organism of interest. This means that we can apply the genetic information gained from studies on model organisms to nontraditional models for which genomic information is not available. Genes could become candidates based on genetic studies in other species (i.e. when polymorphism has already been identified and associated with behavioral variation, Fitzpatrick et al., 2005), or because a physiological pathway leading to behavior of interest is well-understood, suggesting hypotheses about which genes to examine. For example, the neuroendocrine mechanisms underlying circadian rhythms are well described for several mammalian species (Reppert and Weaver, 2002). Therefore, we can ask whether the expression or structure of genes along those pathways differ among individuals or among groups. Candidate gene expression approaches typically involve measuring the abundance of mRNA transcript in a particular tissue (usually brain, in the case of behavior) using quantitative real-time PCR or Northern blots. One of the attractions of the candidate gene approach is that detailed genomic information on the species is not necessary, therefore this technique can be applied to non-model organisms by designing degenerate primers based on other species (Fitzpatrick et al., 2005). Another attraction of the candidate gene approach is that unlike quantitative genetic approaches, it does not require complicated breeding designs or data on the relationships among individuals – comparisons can be made across individuals of unknown relatedness because the genes are being measured directly. Therefore this approach is suitable for organisms that do not readily breed in the lab. Summaries of using the candidate gene approach for studying behavior are welldescribed elsewhere, see (Choleris et al., 2004; Fitzpatrick et al., 2005; Robinson et al., 8 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 2005). Notable examples include work on the for gene and foraging behavior (Debelle et al., 1987; Debelle and Sokolowski, 1989; Debelle et al., 1993; Graf and Sokolowski, 1989; Ingram et al., 2005; Osborne et al., 1997; Pereira and Sokolowski, 1993), and the ‘fruitless’ gene and mating behavior in Drosophila (Kimura et al., 2005; Villella et al., 2005) and the promoter region of the vasopressin gene and parental behavior (Hammock and Young, 2002). The candidate gene approach for studying the genetics of fish behaviour is a relatively unexplored but promising future research direction. While the candidate gene approach is very attractive and has great appeal especially for nonmodel organisms, there are some drawbacks. First, candidate gene studies are biased; what if the ultimate source of genetic differences between groups lies further up or downstream of the particular candidate gene? One approach to this issue is to choose a pathway that is probably associated with the behavior and look at gene expression at several points in the pathway. Second, candidate gene studies are purely correlative. Concluding that the gene is really associated with the behavior requires further experimentation (described below). Perhaps most seriously, candidate gene approaches work best when the trait is influenced by just few genes of major effect, which is probably the exception rather than the rule for behavioral traits (Boake, 1994; Boake et al., 2002). Genomics ‘Genomics’ refers to the study of the structure, content and evolution of genomes, including the analysis of the expression and function of genes and proteins (Gibson and Muse, 2002). What distinguishes genomics from other branches of genetics is that it looks at the whole genome simultaneously, rather than focusing on one gene at a time. Luckily, doing genomics does not require a full genome sequence, and there are already several good examples of applying whole-genome approaches to nonmodel organisms (Charlesworth et al., 2001; Feder and Mitchell-Olds, 2003) and ecologicallyrelevant traits, including behavior (Robinson et al., 2005). Genomics has been hailed as an opportunity to integrate mechanistic and evolutionary approaches to studying behavior because information about genes provides neuroscientists and behavioral ecologists with a ‘common language’ (Robinson et al., 2005). Quantitative trait locus mapping The goal of quantitative trait locus (QTL) mapping is to find regions of the genome that are associated with variation in a phenotypic trait (reviewed in (Slate, 2005)). Results from QTL analyses can include the number and location of QTL affecting a trait, their effect size, and whether QTL interact with each other. Those regions contain genes influencing the trait. Determining the precise location of genes within a QTL depends on the density of markers in a genome-wide linkage map. The basic procedure for performing a QTL analysis is to correlate polymorphic markers distributed throughout the genome with a phenotype (Slate, 2005). QTL analysis is a very powerful and thorough approach for finding genes. It is unbiased, in that it starts with the phenotype and blindly searches for correlated genes irrespective of their function (unlike the candidate gene approach). However, QTL analysis has several drawbacks and 9 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 might not be suitable for most organisms. First, the approach requires that the investigator is able to perform controlled laboratory crosses or has detailed pedigree information. Second, depending on the generation time of the species, QTL analysis can take a long time (up to 3 years for annual species). Third, the success of the method in finding genes is contingent on the level of detail within the linkage map; the resolution of the map will strongly affect how many genes lie within a QTL. This is a key issue not only for finding genes but also for inferring the effect sizes of a QTL; if a QTL in fact consists of linked genes, the effect size of any one of the genes within that QTL will be overestimated. This is particularly a problem with small sample sizes (the so-called ‘Beavis effect’) (Slate, 2005). QTL with tens to hundreds of thousands of linked genes are not unexpected because we know that selection can favor the evolution of gene clustering (discussed below). Finally, QTL studies are especially suited for studying extreme, discrete variation rather than continuously varying traits. As the majority of behaviors of interest to behavioral ecologists are continuously-distributed, QTL analyses might not be generally useful. However, QTL analysis is well-suited for studying categorical behaviors which probably have a simple genetic basis such as disperse/no disperse, or alternative mating types (Liu, 1997). An important factor to keep in mind is that even if there is evidence that the behavior of interest is heritable and amenable to evolutionary analyses, that does not necessarily mean that the behavior is going to be mappable because a heritability of 0.75 could reflect the action of just one major gene accounting for 75% of the variation, which could be easily mapped, or of 75 different genes, each accounting for 1% of the variation, which would be difficult to map. Despite these caveats, the QTL approach has been successfully applied to study behavioral variation, mostly in mouse, honeybees and drosophila (Anholt and Mackay, 2004; Flint, 2003; Flint and Mott, 2001; Flint et al., 2005; Gleason et al., 2002; Henderson et al., 2004; Hitzemann et al., 2003; Hunt et al., 1999; Hunt et al., 1998; Macdonald and Goldstein, 1999; Mackay, 2004; Mackay et al., 2005; Page et al., 2000; Rueppell et al., 2006; Rueppell et al., 2004; Ruppell et al., 2004; Shaw and Parsons, 2002; Turri et al., 2004; Yalcin et al., 2004), with one recent fish study. For example, wild zebrafish (Danio rerio) are more bold toward novel objects and do not shoal as readily as domesticated zebrafish and those behavioral differences can be mapped (Wright et al., 2006). Wright et al. crossed wild and domesticated zebrafish, intercrossed the resulting F1s, and measured the F2s for behavior. They genotyped 84 F2s at 66 loci, and measured their shoaling tendency and boldness toward a novel object, and found three QTLs that were correlated with behavioral differences. One of the most surprising but consistent results to come out of the spate of QTL studies over the past 10 years is that genes of major effect appear to be common, e.g. (Bradshaw et al., 1998). However, genes of major effect are also the ones that are easiest to detect, the so-called ‘low lying fruit’ (Walsh, 2001), so it is premature to conclude that Mendel was right all along. Microarray approaches One of the most exciting new techniques for studying the genetics of behavior involves using microarrays to monitor the expression of thousands of genes 10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 simultaneously. A microarray consists of a glass slide spotted with oligonucleotides or cDNAs of known genes. The experimenter can measure the amount of mRNA present in a particular tissue by applying RNA from a sample onto the plate and then quantifying how much of the sample binds to each oligo (see (Churchill, 2002) for more details on experimental design of microarray experiments). Behavioral experiments using microarrays might compare gene expression following different kinds of experiences (Lawniczak and Begun, 2004), across different behavioral types (Atlantic salmon, Aubin-Horth et al., 2005; Aubin-Horth et al., 2005; see also Whitfield et al., 2003), or among individuals from different populations or strains (reviewed in (Hofmann, 2003; Paratore et al., 2006; Ranz and Machado, 2006)). Comparing the coordinated expression of thousands of genes simultaneously is revealing several unexpected insights. For instance, using cDNA microarrays, Giger et al compared the expression of 900 genes between three different groups: migratory brown trout populations, non-migratory brown trout populations and one population of Atlantic salmon (Giger et al., 2006). They also determined the evolutionary relatedness among these groups using microsatellite markers. Among the brown trout populations, they found that genetic relatedness had little effect on the pattern of gene expression; populations that were more closely related to each other did not show more similar patterns of gene expression relative to distantly-related populations. Instead, the biggest source of variation was life history strategy: 45% of the total variability in gene expression could be attributed to differences between migratory and non-migratory forms. In fact, 268 out of the 900 genes differed in expression between the migratory and non-migratory forms. However, when the other species (Atlantic salmon) was included in the analysis, more than half of the total variance in gene expression was explained by genetic differences between groups, while less than 3% was explained by life-history differences. Therefore, these results suggest that interspecific differences in gene expression are mostly attributable to genetic differences between the species, but intraspecific differences in gene expression is mostly attributable to ecological, life history and behavioral events that are experienced by individuals. The study by Giger et al. is a particularly good example of a hypothesis-driven microarray experiment which did much more than simply describe patterns of gene expression. The experimental design allowed the authors to test for the relationship between genetic relatedness and gene expression similarity, while at the same time comparing gene expression patterns within and between species. The strengths of a microarray approach are that it is: 1) unbiased, in that it is scanning the entire genome for relevant genes rather than cherry-picking one gene at a time; 2) open-ended and therefore good for finding candidate genes; 3) considers the coordinated action of the entire genome, which is probably good for polygenic behavioral traits. Another argument for using microarrays is that they are efficient – each microarray is self-contained experiment, so comparing relative expression across genes is straightforward. Microarrays are also good for nonmodel organisms in which it is not efficient to use traditional forward genetic approaches (Feder and Mitchell-Olds, 2003; Robinson et al., 2005). However, there are several drawbacks to the microarray approach. The first, and most obvious, is that it requires a microarray. This limitation might not be as serious as it appears, though, because several studies have successfully employed microarray 11 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 technology developed for other species on a species of interest, such as Atlantic salmon (Aubin-Horth et al., 2005) and rainbow trout (Sneddon et al., 2005), for other taxa see (Hofmann, 2003; Hofmann et al., 1999; Renn et al., 2004), and progress is being made toward developing universal microarrays (Roth and al, 2004), but see (Karssen, 2006). This is an active area of research and at the time of this writing it is too early to determine if there are any general guidelines regarding the expected error rate and bias toward highly conserved genes (Vasemägi and Primmer, 2005). The next big step after a microarray experiment is to show the evolutionary and ecological significance of variation in transcript levels (note that the candidate gene expression approach has the same problem). Differences in transcript abundance could be a consequence, rather than a cause of differences in behavior. Moreover, identifying the causative genetic polymorphisms underlying the difference in gene expression can be difficult (Vasemägi and Primmer, 2005). That is, the real root of the variation could be occurring upstream in the pathway. This is an active area of research within genomics (‘genetical genomics’ (Jansen and Nap, 2001; Ranz and Machado, 2006; Vasemagi and Primmer, 2005)). Another consideration to bear in mind when using micorarrays to study behavior is that the timing of measurement of gene expression is critical. Finally, another thing to consider is that there is often considerable variation in gene expression across even genetically-identical individuals (Oleksiak et al., 2002; Pritchard et al., 2006). Other whole-genome approaches There are several other whole-genome tools for studying the genetic basis for quantitative variation that are accessible to organisms without a complete genome sequence. These approaches are well-described elsewhere (Gibson and Muse, 2002) and the technology is rapidly changing, so they will be discussed just briefly here. If a microarray is not available, other gene expression comparisons such as subtractive hybridization and RNA differential display are useful for identifying differentially expressed genes (Gibson and Muse, 2002). For example, Sneddon et al. (2005) used suppression subtraction hybridization to create a cDNA library that contained genes that were differentially expressed between rainbow trout of differing dominance status. They then used this cDNA library to construct a microarray to compare gene expression in dominant and subordinate trout. It has become obvious to both scientists and laypeople that having a complete genome sequence is not ‘the answer’. The fact that organisms, including ourselves, share so much of the same DNA code with phenotypically and phylogenetically divergent organisms drew attention to the inadequacy of the precise sequence itself in differentiating groups or explaining biological variation. So what, then, is the big deal about having a whole genome sequence? There are many reasons, but the basic answer is that it makes doing molecular biology a lot easier. Finding genes, for example, is greatly facilitated with a complete genome sequence; without a genome sequence, localizing a particular gene from within a QTL can require laborious chromosome walking, which can be done in silico, or electronically, if the full sequence is available. Other benefits of a whole genome sequence are that it becomes straightforward to design primers for amplifying a gene of interest, and it allows quick 12 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 identification of microsatellite or SNP (single nucleotide polymorphism) markers (Morin et al., 2004). Another advantage is that with a whole genome sequence and a computer, it is easy to determine if a candidate gene has a homolog in your species, and where the gene is located in the genome. ‘Hot topics’ in the genetics of behaviour In this section, I draw attention to two particularly exciting topics in the genetics of behavior. Admittedly, this is a very biased selection, but includes a representative topic from two general categories. The first category is longstanding issues in animal behavior to which a genomic approach has a lot to offer (the role of the environment). The second category is current ‘hot’ topics in behavioral ecology that could benefit from a genetic approach (behavioral syndromes). The environment While this chapter has focused on genetic effects, like many complex traits, the expression of many behaviors is sensitive to environmental conditions, so a complete genetic understanding of behavior requires a formulation that includes environmental inputs over developmental time. A purely genes-based approach to understanding the evolution of behavior clearly does not have all the answers (Hofmann, 2003) because the environment affects when and where genes are expressed. One of the most promising offerings of the genomic revolution for animal behavior is a potential tool for integrating the effects of genetics and the environment on behavior (GxE). Gene expression (‘transcriptomics’) might be a key integrative link between genetic and environmental cues because it reflects the actions of both genetic and environmental inputs while at the same time feeding back on them (e.g. via genomic imprinting and epigenetic programming (Weaver et al., 2004). Another recurring theme in the genetics of complex traits is that GxE interactions are ubiquitous and important (Mackay, 2004; Merila and Sheldon, 1999). Whereas plasticity occurs when the environment affects the expression of the phenotype, GxE interactions go one step further by indicating that there is genetic variation for responsiveness to the environment. In other words, GxE interactions occur when different genotypes respond differently to the same set of environments. While GxE interactions can be a source of annoyance and noise to strictly genetic studies because they can make it more difficult to find genes, for those of us interested in plasticity, the fact that genetic studies are turning up more and more evidence for them is good news. That is because GxE interactions reflect genetic variation for plasticity. Therefore a GxE interaction indicates that plasticity, as a trait, might respond to selection. A promising strategy for investigators interested in both genetics and plasticity is to conduct experiments that examine both genetic and environmental effects simultaneously rather than separately. A researcher might, for example, utilize an experimental design in which full sibs are reared in different kinds of environments, or simultaneously compare gene expression in different genetic groups (e.g. strains or populations) as well as under different environmental conditions (e.g. social/nonsocial, predator/no predator, etc). 13 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Experiments which examine both genetic and environmental effects simultaneously have two additional advantages. The first is that they are efficient – they can test three hypotheses (the role of environment, genetics and GxE) rather than just one. Second, varying the environment can increase the chances of detecting a genetic effect. That is, in many cases, the effect of genotype is only apparent in certain kinds of environments. In fact, in some cases it can be more accurate to view environmental effects as a generator of genetic variation rather than noise; environmental stimuli can uncover parts of the norm of reaction that are only expressed under certain conditions (West-Eberhard, 2003). There are several excellent examples of GxE interactions for behavioral traits. Relatively low-tech experiments on fishes which compared the effect of different environmental conditions on genetically-differentiated populations have revealed that sticklebacks and minnows from ‘high predation’ localities are more responsive to experience with predators relative to stickleback and minnows from low predation localities (Huntingford et al., 1994; Magurran, 1990). Behavioral syndromes There is a long history of studying the genetics of several traits simultaneously in evolutionary biology. Multivariate approaches to selection analyses using the G matrix explicitly take correlations among traits into account when inferring the past or future course of evolution (Arnold, 1994). In addition, artificial selection experiments have consistently produced correlated responses to selection on other traits (Fuller et al., 2005). Therefore, the fact that traits do not evolve independently of one another is widely appreciated in evolutionary biology. Recently, the significance of this insight for animal behavior has come to the forefront. Animal behavior as a discipline has tended to emphasize distinctions between different functional categories of behavior rather than the overlap between them (the table of contents of Animal Behavior textbooks illustrates this point well). However, there is accumulating evidence that behaviors tend to be correlated across different contexts, and form behavioral syndromes. In fish, behavioral syndromes have been found, for example, in three-spined stickleback (Bell, 2005; Bell and Stamps, 2004), brown trout Salmo trutta (Sundström et al, 2004) and pumpkinseed sunfish Lepomis gibbosus, Wilson et al. 1993; 1998; for other taxa see Carere et al., 2001; Dall et al., 2004; Dingemanse et al., 2004; Dingemanse et al., 2002; Drent et al., 2003; Gosling, 2001; Hedrick, 2000; Johnson and Sih 2005; Koolhaas et al., 1999; Reale and Festa-Bianchet, 2003; Reale et al., 2000; Sih et al., 2004; Sih et al., 2004; Stamps, 2003; Verbeek et al., 1996; ; Wilson et al., 1994; ). One of the reasons why behavioral syndromes have been attracting attention is because correlations among behaviors might constrain the ability of behaviors to change and evolve independently of one another (Sih et al., 2004; Sih et al., 2004). Within the lifetime of an individual, behavioral syndromes might produce limited plasticity; if individuals have a ‘tendency’ to behave a certain way in several different situations, and different levels of behavior are favored in different circumstances, then individuals might not express the optimal behavior in every situation. For example, an aggressive individual might do well during competition for resources but might be overly aggressive toward offspring (Ketterson and Nolan, 1999). Therefore behavioral syndromes might be able to 14 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 explain why animals do not always express the optimal behavior for any given situation (Johnson, 2003). If different behaviors are influenced by the same genes (pleiotropy), those genes might constrain optimal behavior. Over evolutionary time, genetic correlations between behaviors might prevent single behaviors from evolving independently of each other. While there is good evidence that traits, including behavioral ones, often occur together in packages, the extent to which those links constrain adaptation is less clear (Bell, 2005). Much has been written about the extent to which correlations among traits reflect the product of natural selection or if they are a constraint on it (Pigliucci and Preston, 2004). On one hand, genetic correlations might constrain a trait’s evolution. If two traits are influenced by the same gene (pleiotropy), then this could result in a tradeoff between the two traits. On the other hand, rigorous selection experiments have revealed that even tightly-linked, genetically correlated traits can be uncoupled (Beldade et al., 2002; Weber, 1992). One way to test for the genetic constraints is to compare the relationship between traits in different kinds of populations. If the relationship between traits varies across populations, that suggests that genetic correlations are not constraining the number of combinations possible and that the different combinations might represent adaptations to different selective conditions. I applied this reasoning to a behavioral syndrome in order to test whether behavioral syndromes can act as an evolutionary constraint. Previous work had shown that threespined sticklebacks could be characterized by the ‘boldness aggressiveness behavioral syndrome’: individuals sticklebacks that were more bold toward predators were also more aggressive toward conspecifics (Huntingford, 1976). However, different populations of sticklebacks vary, on average, in boldness and aggressiveness, and much of the variation across populations has been attributed to differences in predation pressure (reviewed in (Huntingford, 1994). Using full sib and parent-offspring resemblance and the animal model, I found that the genetic architecture underlying boldness and aggressiveness differed in two different populations of sticklebacks (Bell, 2005). Specifically, boldness and aggressiveness were positively genetically correlated in one population but not the other one. Ecological comparisons between the two populations suggests that the population in which the syndrome occurs is subject to strong selection by bird and fish predators. Therefore this study provides support for the hypothesis that some behavioral syndromes might be the result of selection, rather than a constraint on it. In fact, the genetic correlation between boldness and aggressiveness in some populations might reflect a history of correlational selection which has favored particular trait value combinations. Correlational selection will result in linkage disequilibrium between the co-selected genes (Lande and Arnold, 1983). Understanding the mechanisms underlying genetic correlations might be able to disentangle the ‘constraint’ and ‘adaptation’ hypotheses about the significance of behavioral syndromes. If the genetic correlation is due to linkage disequilibrium, and possibly a product of correlational selection, the genetic correlation will break down in hybrids (Conner, 2002), suggesting that the genetic correlation can be uncoupled. If the genetic correlation is preserved in hybrids, that suggests the two traits are affected by the same genes and the genetic correlation will be more difficult to modify through evolutionary time. In addition to simple crossing 15 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 experiments, access to the actual genes as opposed to just their statistical consequences will shed some light on the extent to which genetic correlations can act as a constraint. Finally, while behavioral syndromes have drawn attention to correlated behaviors, an even broader view is that all aspects of the phenotype (behavior, morphology, physiology, life history) function as an integrated unit and are best studied as such (Pigliucci and Preston, 2004). Therefore, genetic studies might be most successful when they measure several aspects of the phenotype simultaneously rather than dissecting each piece individually. Conclusions Behavioral ecologists will have an important role to play in the genomic revolution. Understanding a species’ natural variation in behavior can help make sense of the wealth of genomic variation that confronts us. This is especially an issue for model organisms such as zebrafish, which have been heralded as a rising model system for studying the genetics of behavior (Gerlai, 2003; Robison and Rowland, 2005; Wright et al., 2006; Wright et al., 2003), yet we know very little about the natural behavior of zebrafish in the field. While they might not be particularly charismatic, zebrafish offer tremendous opportunities for a young behavioral ecologist trying to choose an organism to study. Studying natural variation in behavior and its ecological relevance for model systems such as zebrafish is important to genetic studies for several reasons. First, basic ethological information about what the animal does in its natural environment is critical for determining what traits are relevant to the animal. Second, knowing how an organism interacts with its environment is key to determine how to measure the behavior in a laboratory environment, such as which cues or experimental setups will most efficiently elicit the behavior of interest. Third, knowing what the animal does in its natural environment and how it varies according to different selective pressures gives hints as to whether the variation is adaptive and if it might have been subject to past or future selection. Finally, natural variation in behavior might be a better approximation to the kind of variation of interest to human health and disease (Koolhaas, 2006). That is, unlike behavioral variation in laboratory animals which have been protected from ecologically relevant selective forces, natural variation in behavior can represent adaptive solutions to particular selective challenges. Behavioral ecologists can also help direct genetic studies in hypothesis-driven research directions. An understanding of the ecology of the species in question can help point to particularly interesting biological issues faced by the organism. Understanding the natural history of the species will also help interpret the results of genetic manipulations. For example, if we know the ecological significance of the traits that are affected by knocking out genes or by blocking their expression using RNAi, that could suggest functional reasons why the traits are correlated in the first place. Therefore there is great scope for collaboration between behavioral ecologists and their molecular colleagues. If this chapter has not scared off the faint-of-heart, I hope that it has convinced researchers who were not thinking of including a genetic component in their research program to reconsider. But if they should choose to embark on such a mission, a 16 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 recurring theme in discussions of the genetics of complex traits (Boake et al., 2002; Feder and Mitchell-Olds, 2003; Vasemagi and Primmer, 2005) and in this chapter is that no single genetic approach has all the answers, and research programs which are integrative and multi-pronged are the way forward. Research strategies which approach the question from different angles and which use complementary techniques that give different types of data are probably the best way to approach the genetics of complex traits. Of course no single research program can do it all; it would be logistically impossible for a single PhD project to perform experiments which look at a range of environments, which considers behavioral, physiological and morphological traits, and which carries out QTL, candidate gene, microarray experiments all at once. However, research strategies that take just two approaches complementary approaches will be fruitful. For example, a study might carry out a QTL analysis in parallel with the analysis of candidate gene expression, and then look to see if the informative candidate genes lie within QTL (Letwin, 2006). Similarly, doing experiments which explicitly study both genetic and environmental effects simultaneously, and which measure entire suites of traits rather than just one trait at a time will be the most lucrative. In 1984, when Grafen made his original ‘gambit’, we knew very little about the genetics of complex phenotypes. Grafen’s deal was provisional, revocable when the assumption that the genetic basis to behavior is simple was no longer tenable and when genes were more within the reach of behavioral ecologists. Since then, we have learned that the genetics of complex traits such as behavior are far more complicated than he originally envisioned (Mackay, 2004). In addition, the genetic tools necessary to approach this complexity are becoming more available. Luckily, approaching the genetics of complex traits is within the grasp of behavioral ecologists and a more thorough understanding of genetic mechanisms will make our studies richer. Acknowledgements I thank Tim Caro for challenging me with the phenotypic gambit, Katie Peichel for informative conversations about QTL analysis, Beverly Ajie, Jason Watters, Ripan Malhi and Niels Dingemanse for comments on the chapter. References Adams, C. E., C. Woltering and G. Alexander. 2003. Epigenetic regulation of trophic morphology through feeding behaviour in Arctic charr, Salvelinus alpinus. Biological Journal of the Linnean Society 78(1): 43-49. Alvarez, D. and A. G. Nicieza. 2003. Predator avoidance behaviour in wild and hatcheryreared brown trout: the role of experience and domestication. Journal of Fish Biology 63(6): 1565-1577. Anholt, R. R. H. and T. F. C. Mackay. 2004. Quantitative genetic analyses of complex behaviours in Drosophila. Nature Reviews Genetics 5(11): 838-849. Arnold, S. J. 1994. Multivariate inheritance and evolution: a review. In: Quantitative Genetics and Behavioral Evolution. C. R. B. Boake (Ed.). University of Chicago Press. Chicago, pp. 17-48. 17 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 Aubin-Horth, N., C. R. Landry and H. A. Hofmann. 2005. Alternative life histories shape brain gene expression profiles in males of the same population. Proceedings of the Royal Society B-Biological Sciences 272(1573): 1655-1662. Aubin-Horth, N., B. H. Letcher and H. A. Hofmann. 2005. Interaction of rearing environment and reproductive tactic on gene expression profiles in Atlantic salmon. Journal of Heredity 96(3): 261-278. Bakker, T. C. M., 1993. Positive genetic correlation between female preference and preferred male ornament in sticklebacks. Nature 363: 255-257. Beldade, P., K. Koops and P. M. Brakefield. 2002. Developmental constraints versus flexibility in morphological evolution. Nature 416: 844-847. Bell, A. M. 2005. Differences between individuals and populations of threespined stickleback. Journal of Evolutionary Biology 18: 464-473. Bell, A. M. and J. A. Stamps. 2004. The development of behavioural differences between individuals and populations of stickleback. Animal Behaviour 68: 1339-1348. Bell, M. A. and S. A. Foster. 1994. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press, Oxford. Boake, C. R. B. 1994. Quantitative Genetic Studies of Behavioral Evolution. University of Chicago Press, Chicago. Boake, C. R. B., S. J. Arnold, F. Breden, L. M. Meffert, M. G. Ritchie, B.J. Taylor, J.B. Wolf and A. J. Moore. 2002. Genetic tools for studying adaptation and the evolution of behavior. American Naturalist 160: S143-S159. Both, C. and M. E. Visser. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411: 296-298. Boughman, J. W. 2002. How sensory drive can promote speciation. Trends in Ecology and Evolution 17: 571-577. Bradshaw, H. D. J., K. G. Otto, B. E.Frewen, J. K. McKay and D. W. Schemske.1998. Quantitative trait loci affecting differences in floral morphology between two species of monkeyflower (Mimulus). Genetics 149: 367-82. Broadhurst, P. L. and J. L. Jinks. 1979. What genetical architecture can tell us about natural selection on behavioral traits. In: The Genetics of Behavior. J. H. F. van Abeelen (Ed.). Elsevier. New York, pp. 43-63. Brooks, D. R. and D. A. McLennan. 1991. Phylogeny, Ecology and Behavior: A Research Program in Comparative Biology. University of Chicago Press, Chicago. Brooks, R., and V. Couldridge. 1999. Multiple sexual ornaments coevolve with multiple mating preferences. American Naturalist 154: 37-45. Carere, C., D. Welink, P. J. Drent, J. M. Koolhaas and T.G. Groothuis . 2001. Effect of social defeat in a territorial bird selected for different coping styles. Physiology & Behavior 73: 427-433. Chapman, L. J., F. Galis and J. Shinn. 2000. Phenotypic plasticity and the possible role of genetic assimilation: Hypoxia-induced trade-offs in the morphological traits of an African cichlid. Ecology Letters 3(5): 387-393. Charlesworth, D., B. Charlesworth and G. A. T. McVean. 2001. Genome sequences and evolutionary biology, a two-way interaction. Trends in Ecology & Evolution 16(5): 235-242. 18 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Choleris, E., M. Kavaliers and D. W. Pfaff. 2004. Functional genomics of social recognition. Journal of Neuroendocrinology 16: 383-389. Churchill, G. A. 2002. Fundamentals of experimental design for cDNA microarrays. Nature Genetics 32: 490 - 495. Conner, J. K. 2002. Genetic mechanisms of floral trait correlations in a natural population. Nature 420: 407-410. Colosimo, P. F., K. E. Hosemann, S. Balabhadra, J. Guadalupe Villarreal, M. Dickson, J. Grimwood, J. Schmutz, R. M. Myers, D. Schluter and D. M. Kingsley. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307: 1928 - 1933. Dall, S. R. X., A. I. Houston and J. M. McNamara. 2004. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecology Letters 7(8): 734-739. Debelle, J. S., A. J. Hilliker and M. B. Sokolowski. 1987. Genetic localization of the rover sitter larval foraging polymorphism in Drosophila melanogaster. Behavior Genetics 17(6): 620-620. Debelle, J. S. and M. B. Sokolowski. 1989. Rover sitter foraging behavior in Drosophila melanogaster - Genetic localization to chromosome 2l using compound autosomes. Journal of Insect Behavior 2(3): 291-299. Debelle, J. S., M. B. Sokolowski and A. J. Hilliker. 1993. Genetic analysis of the foraging microregion of Drosophila melanogaster. Genome 36(1): 94-101. Dingemanse, N. J., C. Both, P. J. Drent and J. M. Tinbergen. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society of London - Series B: Biological Sciences 271(1541): 847-852. Dingemanse, N. J., C. Both, P. J. Drent, K. van Oers and A. J. van Noordwijk. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Animal Behaviour 64(6): 929-938. Dobzhansky, T. 1937. Genetics and the Origin of Species. Columbia University Press, New York. Drent, P. J., K. Van Oers and A. J. van Noordwijk. 2003. Realised heritability of personalities in the great tit (Parus major). Proceedings of the Royal Society of London, Series B. 270: 45-51. Endler, J. A. 1995. Multiple-trait coevolution and environmental gradients in guppies. Trends in Ecology & Evolution 10(1): 22-29. Falconer, D. S. 1989. Introduction to Quantitative Genetics, 2nd edition. Longman, New York. Feder, M. E. and T. Mitchell-Olds. 2003. Evolutionary and ecological functional genomics. Nature Reviews Genetics 4(8): 651-657. Fitzpatrick, M. J., Y. Ben-Shahar, H. M. Smid, L. E. M. Vet, G. E. Robinson and M. B. Sokolowski. 2005. Candidate genes for behavioural ecology. Trends in Ecology & Evolution 20(2): 96-104. Fitzpatrick, M. J. and M. B. Sokolowski. 2004. In search of food: Exploring the evolutionary link between cGMP-dependent protein kinase (PKG) and behaviour. Integrative and Comparative Biology 44(1): 28-36. Flint, J. 2003. Analysis of quantitative trait loci that influence animal behavior. Journal of Neurobiology 54(1): 46-77. 19 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 Flint, J. and R. Mott. 2001. Finding the molecular basis of quantitative traits: Successes and pitfalls. Nature Reviews Genetics 2(6): 437-445. Flint, J., W. Valdar, S. Shifman and R. Mott. 2005. Strategies for mapping and cloning quantitative trait genes in rodents. Nature Reviews Genetics 6(4): 271-286. Foster, S. A. and J. A. Endler. 1999. Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford University Press, New York. Fuller, R. C., C. F. Baer and J. Travis. 2005. How and when selection experiments might actually be useful. Integrative & Comparative Biology 45: 391-404. Gerlai, R. 2003. Zebra fish: An uncharted behavior genetic model. Behavior Genetics 33(5): 461-468. Gibson, G. and S. V. Muse. 2002. A Primer of Genome Science. Sinauer Associates, Inc., Sunderland, MA. Giger, T., L. Excoffier, P. J. R. Day, A. Champigneulle, M. M. Hansen, R. Powell and C. R. Largiader. 2006. Life history shapes gene expression in salmonids. Current Biology 16(8): R281-R282. Gleason, J. M., S. V. Nuzhdin and M. G. Ritchie. 2002. Quantitative trait loci affecting a courtship signal in Drosophila melanogaster. Heredity 89: 1-6. Gosling, S. D. 2001. From mice to men: what can we learn about personality from animal research? Psychological Bulletin 127: 45-86. Graf, S. A. and M. B. Sokolowski. 1989. Rover sitter Drosophila melanogaster larval foraging polymorphism as a function of larval development, food patch quality, and starvation. Journal of Insect Behavior 2(3): 301-313. Grafen, A. 1984. Natural selection, kin selection and group selection. In: Behavioral Ecology: An Evolutionary Approach (2nd edition). J. R. Krebs and N. B. Davies (Ed.). Blackwell Science. Oxford, pp. Grant, P. R. and B. R. Grant. 1995. Predicting microevolutionary responses to directional selection on heritable variation. Evolution 49: 241-251. Grether, G. F., D. F. Millie, M. J. Bryant, D. N. Reznick and W. Mayea. 2001. Rain forest canopy cover, resource availability, and life history evolution in guppies. Ecology 82(6): 1546-1559. Hammock, E. A. D. and L. J. Young. 2002. Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour. European Journal of Neuroscience 16(3): 399-402. Hedrick, A. V. 2000. Crickets with extravagant mating songs compensate for predation risk with extra caution. Proceedings of the Royal Society Biological Sciences, Series B 267(1444): 671-675. Helfman, G. S., B. B. Collette and D. E. Facey. 2000. The Diversity of Fishes. Blackwell, Malden, MA. Henderson, N. D., M. G. Turri, J. C. DeFries and J. Flint. 2004. QTL analysis of multiple behavioral measures of anxiety in mice. Behavior Genetics 34(3): 267-293. Hitzemann, R., B. Malmanger, C. Reed, M. Lawler, B. Hitzemann, S. Coulombe, K. Buck, B. Rademacher, N. Walter, Y. Polyakov, J. Sikela, B. Gensler, S. Burgers, R. W. Williams, K. Manly, J. Flint and C. Talbot. 2003. A strategy for the integration of QTL, gene expression, and sequence analyses. Mammalian Genome 14(11): 733-747. 20 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Hoekstra, H. E., K. E. Drumm and M. W. Nachman. 2004. Ecological genetics of adaptive color polymorphism in pocket mice: geographic variation in selected and neutral genes. Evolution 58(6): 1329-1341. Hofmann, H. A. 2003. Functional genomics of neural and behavioral plasticity. Journal of Neurobiology 54(1): 272-282. Hofmann, H. A., M. E. Benson and R. D. Fernald. 1999. Social status regulates growth rate: Consequences for life-history strategies. Proceedings of the National Academy of Sciences of the United States of America 96(24): 14171-14176. Houde, A. E., and Endler, J. A., 1990. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science 248: 1405-1408. Houde, A. E., 1994. Effect of artificial selection on male color patterns on mating preference of female guppies. Proceedings of the Royal Society of London Series B-Biological Sciences 256: 125-130. Houle, D. 1992. Comparing evolvability and variability in quantitative traits. Genetics 130: 195-204. Houle, D., B. Morikawa and M. Lynch. 1996. Comparing mutational variabilities. Genetics 143: 1467-1483. Hunt, G. J., A. M. Collins, R. Rivera, R. E. Page and E. Guzman-Novoa. 1999. Quantitative trait loci influencing honeybee alarm pheromone levels. Journal of Heredity 90(5): 585-589. Hunt, G. J., E. Guzman-Novoa, M. K. Fondrk and R. E. Page. 1998. Quantitative trait loci for honey bee stinging behavior and body size. Genetics 148(3): 1203-1213. Huntingford, F. A. 2004. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. Journal of Fish Biology 65: 122-142. Huntingford, F. A., P. J. Wright and J. F. Tierney. 1994. Adaptive variation and antipredator behaviour in threespine stickleback. In: The Evolutionary Biology of the Threespine Stickleback. M. A. Bell and S. A. Foster (Ed.). Oxford. Oxford, pp. 277-295. Huntingford, F. A., 1976. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback. Animal Behaviour 24: 245-260. Ingram, K. K., P. Oefner and D. M. Gordon. 2005. Task-specific expression of the foraging gene in harvester ants. Molecular Ecology 14(3): 813-818. Jaenisch, R. 1988. Transgenic animals. Science 240(4858): 1468-1474. Jansen, R. C. and J. P. Nap. 2001. Genetical genomics: the added value from segregation. Trends in Genetics 17: 388-391. Johnson, J., and A. Sih. 2005. Pre-copulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behavioral Ecology Sociobiology 58, 390-396. Jones, A. G., S. J. Arnold and R. Borger. 2003. Stability of the G-matrix in a population experiencing pleiotropic mutation, stabilizing selection, and genetic drift. Evolution 57(8): 1747-1760. Karssen, A. M., J. Z. Li, S. Her, P. D. Patel, F. Meng, S. J. Evans, M. P. Vawter, H. Tomita, P. V. Choudary, W. E. Bunney, E. G. Jones, S. J. Watson, H. Akil, R. M. Myers, A. F. Schatzberg and D. M. Lyons. 2006. Application of microarray technology in primate behavioral neuroscience research. Methods 38: 227-234. 21 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Ketterson, E. D. and V. Nolan. 1999. Adaptation, exaptation, and constraint: A hormonal perspective. American Naturalist 154(SUPPL.): S4-S25. Ketterson, E. D., V. Nolan, M. J. Cawthorn, P. G. Parker and C. Ziegenfus. 1996. Phenotypic engineering: Using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis 138(1): 70-86. Kimura, K. I., M. Ote, T. Tazawa and D. Yamamoto. 2005. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438(7065): 229-233. Koolhaas, J. M., S. M. Korte, S. F. DeBoer, B. J. Van Der Vegt, C. G. Van Reenen, H. Hopster, I. C. De Jong, M. A. W. Ruiz and H. J. Blokhuis. 1999. Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews 23(7): 925-935. Koolhaas, J. M., De Boer, S. F., and Buwalda, B., 2006. Stress and adaptation. Current Directions in Psychological Science 15: 109-112. Kruuk, L. E. B. 2003. Estimating genetic parameters in natural populations using the 'animal model'. Philosophical Transactions of the Royal Society of London, Series B 359: 873-890. Lahti, K., A. Laurila, K. Enberg and J. Piironen. 2001. Variation in aggressive behaviour and growth rate between populations and migratory forms in the brown trout, Salmo trutta. Animal Behaviour 62: 935-944. Lande, R. 1979. Quantitative genetic analysis of multivariate evolution applied to brain:body size allometry. Evolution 33: 402-416. Lande, R. and S. J. Arnold. 1983. The measurement of selection on correlated characters. Evolution 37: 1210-1226. Lawniczak, M. K. N. and D. J. Begun. 2004. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females Genome 47: 900-910. Lepage, O., O. Overli, E. Petersson, T. Jarvi and S. Winberg. 2000. Differential stress coping in wild and domesticated sea trout. Brain Behavior and Evolution 56(5): 259-268. Lessels, C. M. and P. T. Boag. 1987. Unrepeatable repeatabilities: a common mistake. The Auk 104: 116-121. Letwin, N. E., N. Kafkafi, Y. Benjamini, C. Mayo, B. C. Frank, T. Luu, N. H. Lee, G. I. Elmer. 2006. Combined application of behavior genetics and microarray analysis to identify regional expression themes and gene-behavior associations. The Journal of Neuroscience 26: 5277-5287. Lewontin, R. C., S. Rose and L. J. Kamin. 1984. Not In Our Genes: Biology, Ideology, and Human Nature. Pantheon Books, New York. Liu, B.-H. 1997. Statistical genomics: linkage, mapping and QTL analysis. CRC, New York. Losos, J. B., D. A. Creer, D. Glossip, R. Goellner, A. Hampton, G. Roberts, N. Haskell, P. Taylor and J. Ettling. 2000. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54: 310-315. Lynch, M. and B. Walsh. 1998. Genetics and analysis of quantitative traits. Sinauer Asssociates, Sunderland, MA. Macdonald, S. J. and D. B. Goldstein. 1999. A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics 153(4): 1683-1699. 22 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Mackay, T. F. C. 2004. The genetic architecture of quantitative traits: lessons from Drosophila. Current Opinion in Genetics & Development 14(3): 253-257. Mackay, T. F. C. 2004. The genetic architecture of quantitative traits: lessons from Drosophila. Current Opinion in Genetics & Development 14: 253-257. Mackay, T. F. C., S. L. Heinsohn, R. F. Lyman, A. J. Moehring, T. J. Morgan and S. M. Rollmann. 2005. Genetics and genomics of Drosophila mating behavior. Proceedings of the National Academy of Sciences of the United States of America 102: 6622-6629. Magurran, A. E. 1990. The inheritance and development of minnow anti-predator behaviour. Animal Behavior 39: 834-842. Malavasi, S., V. Georgalas, M. Lugli, P. Torricelli and D. Mainardi. 2004. Differences in the pattern of antipredator behaviour between hatchery-reared and wild European sea bass juveniles. Journal of Fish Biology 65: 143-155. Manzanares, M., H. Wada, N. Itasaki, P. A. Trainor, R. Krumlauf and P. W. H. Holland. 2000. Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature 408(6814): 854-857. Meffert, L. M., S. K. Hicks and J. L. Regan. 2002. Nonadditive genetic effects in animal behavior. American Naturalist 160(Supplement): 198-213. Merila, J. and B. C. Sheldon. 1999. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83: 103-109. Metcalfe, N. B., S. K. Valdimarsson and I. J. Morgan. 2003. The relative roles of domestication, rearing environment, prior residence and body size in deciding territorial contests between hatchery and wild juvenile salmon. Journal of Applied Ecology 40(3): 535-544. Morin, P. A., G. Luikart and R. K. Wayne. 2004. SNPs in ecology, evolution and conservation. Trends in Ecology & Evolution 19: 208-216. Oleksiak, M. F., G. A. Churchill and D. L. Crawford. 2002. Variation in gene expression within and among natural populations. Nature Genetics 32: 261-266. Orzack, S. H. and E. Sober. 1994. Optimality models and the test of adaptationism. American Naturalist 143: 361-380. Osborne, K. A., A. Robichon, E. Burgess, S. Butland, R. A. Shaw, A. Coulthard, H. S. Pereira, R. J. Greenspan and M. B. Sokolowski. 1997. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277(5327): 834-836. Øverli, Ø., T. G. Pottinger, T. R. Carrick, E. Øverli and S. Winberg. 2001. Brain monoaminergic activity in rainbow trout selected for high and low stress responsiveness. Brain Behavior and Evolution 57(4): 214-224. Øverli, Ø., T. G. Pottinger, T. R. Carrick, E. Øverli and S. Winberg. 2002. Differences in behaviour between rainbow trout selected for high- and low-stress responsiveness. Journal of Experimental Biology 205(3): 391-395. Page, R. E., M. K. Fondrk, G. J. Hunt, E. Guzman-Novoa, M. A. Humphries, K. Nguyen and A. S. Greene. 2000. Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. Journal of Heredity 91(6): 474-479. Pakkasmaa, S. and J. Piironen. 2001. Morphological differentiation among local trout (Salmo trutta) populations Biological Journal of the Linnean Society 72: 231239. 23 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Palm, S. and N. Ryman. 1999. Genetic basis of phenotypic differences between transplanted stocks of brown trout. Ecology of Freshwater Fish 8(3): 169-180. Paratore, S., E. Alessi, S. Coffa, A. Torrisi, F. Mastrobuono, S. Cavallaro. 2006. Early genomics of learning and memory: a review. Genes, Brain, & Behavior 5: 209221. Peichel, C. L., K. S. Nereng, K. A. Ohgi, B. L. E. Cole, P. F. Colosimo, C. A. Buerkle, D. Schluter and D. M. Kingsley. 2001. The genetic architecture of divergence between threespine stickleback species. Nature 414(6866): 901-905. Pereira, H. S. and M. B. Sokolowski. 1993. Mutations in the larval foraging gene affect adult locomotory behavior after feeding in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America 90(11): 5044-5046. Petersson, E. and T. Järvi. 2006. Anti-predator response in wild and sea-ranched brown trout and their crosses. Aquaculture 253(1-4): 218-228. Pigliucci, M. and K. A. Preston. 2004. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Oxford University Press, New York. Pottinger, T. G. and T. R. Carrick. 1999. A comparison of plasma glucose and plasma cortisol as selection markers for high and low stress-responsiveness in female rainbow trout. Aquaculture 175(3-4): 351-363. Pottinger, T. G. and T. R. Carrick. 1999. Modification of the plasma cortisol response to stress in rainbow trout by selective breeding. General and Comparative Endocrinology 116(1): 122-132. Pritchard, C., D. Coil, S. Hawley, L. Hsu and P. S. Nelson. 2006. The contributions of normal variation and genetic background to mammalian gene expression. Genome Biology 7: R26. Qvarnström, A., J. E. Brommer. and Gustafsson, L., 2006. Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441: 84-86. Ranz, J. M. and C. A. Machado. 2006. Uncovering evolutionary patterns of gene expression using microarrays. Trends in Ecology & Evolution 21(1): 29-37. Reale, D. and M. Festa-Bianchet. 2003. Predator-induced natural selection on temperament in bighorn ewes. Animal Behaviour 65(3): 463-470. Reale, D., B. Y. Gallant, M. Leblanc and M. Festa-Bianchet. 2000. Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Animal Behaviour 60(5): 589-597. Renn, S. C. P., N. Aubin-Horth and H. A. Hofmann. 2004. Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. BMC Genomics 5: 42. Reppert, S. M. and D. R. Weaver. 2002. Coordination of circadian timing in mammals. Nature 418(6901): 935-941. Robinson, G. E., C. M. Grozinger and C. W. Whitfield. 2005. Sociogenomics: Social life in molecular terms. Nature Reviews Genetics 6(4): 257-U16. Robison, B. D. and W. Rowland. 2005. A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Canadian Journal of Fisheries and Aquatic Sciences 62(9): 2046-2054. 24 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Roff, D. A. and T. A. Mousseau. 1987. Quantitative genetics and fitness: lessons from Drosophila. Heredity 58: 103-118. Roth, M. E., L. Feng, K. J. McConnell, P. J. Schaffer, C. E. Guerra, J. P. Affourtit, K. R. Piper, L. Guccione, J. Hariharan, M. J. Ford, S. W. Powell, H. Krishnaswamy, J. Lane, L. Guccione, G. Intrieri, J. S. Merkel, C. Perbost, A. Valerio, B. Zolla, C. D. Graham, J. Hnath, C. Michaelson, R. X. Wang, B. Ying, C. Halling, C. E. Parman, D. Raha, B. Orr, B. Jedrzkiewicz, J. Liao, A. Tevelev, M. J. Mattessich, D. M. Kranz, M. Lacey, J. C. Kaufman, J. Kim, D. R. Latimer, P. M. Lizardi. 2004. Expression profiling using a hexamer-based universal microarray. Nature Biotechnology 22: 418-426. Ruber, L., R. Britz, H. H. Tan, P. K. L. Ng and R. Zardoya. 2004. Evolution of mouthbrooding and life-history correlates in the fighting fish genus Betta. Evolution 58(4): 799-813. Rueppell, O., S. B. C. Chandra, T. Pankiw, M. K. Fondrk, M. Beye, G. Hunt and R. E. Page. 2006. The genetic architecture of sucrose responsiveness in the honeybee (Apis mellifera L.). Genetics 172(1): 243-251. Rueppell, O., T. Pankiw, D. I. Nielsen, M. K. Fondrk, M. Beye and R. E. Page. 2004. The genetic architecture of the behavioral ontogeny of foraging in honeybee workers. Genetics 167(4): 1767-1779. Ruppell, O., T. Pankiw and R. E. Page. 2004. Pleiotropy, epistasis and new QTL: The genetic architecture of honey bee foraging behavior. Journal of Heredity 95(6): 481-491. Ruzzante, D. E. and R. W. Doyle. 1991. Rapid behavioral changes in medaka caused by selection for competitive and noncompetitive growth. Evolution 45: 1936-1946. Ruzzante, D. E. and R. W. Doyle. 1993. Evolution of social behavior in a resource-rich, structured environment: selection experiments with medaka. Evolution 47: 456470. Schjolden, J., T. Backström, K. G. T. Pulman, T. G. Pottinger and S. Winberg. 2005. Divergence in behavioural responses to stress in two strains of rainbow trout (Oncorhynchus mykiss) with contrasting stress responsiveness. Hormones and Behavior 48(5): 537-544. Schlaepfer, M. A., M. C. unge and P. W. Sherman. 2002. Ecological and evolutionary traps. Trends in Ecology & Evolution 17: 474-480. Shaw, K. L. and Y. M. Parsons. 2002. Divergence of mate recognition behavior and its consequences for genetic architectures of speciation. American Naturalist 159: S61-S75. Sih, A., A. M. Bell and J. C. Johnson. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution 19(7): 372-378. Sih, A., A. M. Bell, J. C. Johnson and R. Ziemba. 2004. Behavioral syndromes: an integrative overview. Quarterly Review of Biology 79: 241-277. Sinervo, B. and R. B. Huey. 1990. Allometric engineering - an experimental test of the causes of interpopulational differences in performance. Science 248(4959): 11061109. Skulason, S., D. L. G. Noakes and S. S. Snorrason. 1989. Ontogeny of trophic morphology in four sympatric morphs of Arctic Charr Salvelinus alpinus in Thingvallavatn Iceland. Biological Journal of the Linnean Society 38(3): 281-301. 25 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Slate, J. 2005. Quantitative trait locus mapping in natural populations: progress, caveats and future directions. Molecular Ecology 2: 363-379. Sneddon, L. U., J. Margareto and A. R. Cossins. 2005. The use of transcriptomics to address questions in behaviour: Production of a suppression subtractive hybridisation library from dominance hierarchies of rainbow trout. Physiological and Biochemical Zoology 78(5): 695-705. Stahl, S. M. and N. Muntner. 2000. Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press, Cambridge. Stamps, J. A. 2003. Tinbergen's fourth question comes of age. Animal Behaviour 66: 113. Steppan, S. J., P. C. Phillips, and D. Houle. 2002. Comparative quantitative genetics: evolution of the G matrix. Trends in Ecology & Evolution 17:329-337. Stirling, D. G., D. Reale and D. A. Roff. 2002. Selection, structure and the heritability of behaviour. Journal of Evolutionary Biology 15: 277-289. Sundström, L. F., E. Petersson, J. Hojesjo, J. I. Johnsson and T. Jarvi. 2004. Hatchery selection promotes boldness in newly hatched brown trout (Salmo trutta): implications for dominance. Behavioral Ecology 15(2): 192-198. Trivers, R. L. 1971. The evolution of reciprocal altruism. The Quarterly Review of Biology 46: 35-57. Turri, M. G., J. C. DeFries, N. D. Henderson and J. Flint. 2004. Multivariate analysis of quantitative trait loci influencing variation in anxiety-related behavior in laboratory mice. Mammalian Genome 15(2): 69-76. Vasemägi, A. and C. R. Primmer. 2005. Challenges for identifying functionally important genetic variation: the promise of combining complementary research strategies. Molecular Ecology 14: 3623-3642. Verbeek, M. E. M., A. Boon and P. J. Drent. 1996. Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133: 945-963. Villella, A., S. L. Ferri, J. D. Krystal and J. C. Hall. 2005. Functional analysis of fruitless gene expression by transgenic manipulations of Drosophila courtship. Proceedings of the National Academy of Sciences of the United States of America 102(46): 16550-16557. Visser, M. E., A. J. van Noordwijk, J. M. Tinbergen, C. M. Lessells. 1998. Warmer springs lead to mistimed reproduction in great tits. Proceedings of the Royal Society Biological Sciences Series B 265: 1867-1870. Walsh, B. 2001. Quantitative genetics in the age of genomics. Theoretical Population Biology 59(3): 175-184. Weaver, I. C. G., N. Cervoni, F. A. Champagne, A. C. D'Alessio, S. Sharma, J. R. Seckl, S. Dymov, M. Szyf and M. J. Meaney. 2004. Epigenetic programming by maternal behavior. Nature Neuroscience 7(8): 847-854. Weber, K. E. 1992. How small are the smallest selectable domains of form? Genetics 130: 345-353. West-Eberhard, M. J. 2003. Developmental Plasticity and Evolution. Oxford University Press, Oxford. Whitfield, C. W., A. M. Cziko and G. E. Robinson. 2003. Gene expression profiles in the brain predict behavior in individual honey bees. Science 302(5643): 296-299. 26 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Wikelski, M. and C. Thom. 2000. Marine iguanas shrink to survive El Nino. Nature 403: 37-38. Wilson, D. S. 1998. Adaptive individual differences within single populations. Philosophical Transactions of the Royal Society of London, Series B 353(1366): 199-205. Wilson, D. S., A. B. Clark, K. Coleman and T. Dearstyne. 1994. Shyness and boldness in humans and other animals. Trends in Ecology and Evolution 11: 442-446. Wilson, D. S., K. Coleman, A. B. Clar and L. Biederman. 1993. Shy-bold continuum in pumpkinseed sunfish: an ecological study of a psychological trait. Journal of Comparative Psychology 107: 250-260. Wright, D., R. Nakamichi, J. Krause and R. K. Butlin. 2006. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behavior Genetics 36(2): 271-284. Wright, D., L. B. Rimmer, V. L. Pritchard, J. Krause and R. K. Butlin. 2003. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio). Naturwissenschaften 90(8): 374-377. Yalcin, B., S. A. G. Willis-Owen, J. Fullerton, A. Meesaq, R. M. Deacon, J. N. P. Rawlins, R. R. Copley, A. P. Morris, J. Flint and R. Mott. 2004. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nature Genetics 36(11): 1197-1202. 27