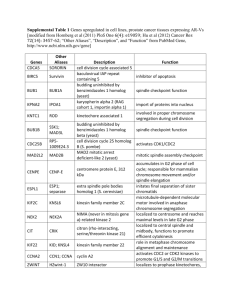
The sequences of siRNAs
advertisement

SUPPPLEMENTAL MATERIAL ADDITIONAL DISCUSSION To further verify whether inhibition of IR-induced ERK1/2 is essential for the inhibition of IR-induced G2/M checkpoint activation, MCF-7 cells were treated with IR first and then incubated with U0126. Under this condition, inhibition of ERK1/2 by U0126 after irradiation had no effect on IR-induced G2/M arrest (data not shown). This result indicates that once the signaling cascade leading to G2/M arrest is activated following IR, continued activation of ERK1/2 is not necessary for the maintenance of the G2/M checkpoint activation in MCF-7 cells following IR treatment. Although JNK kinase is also activated in MCF-7 cells following IR treatment (data not shown), time course experiments indicate that, while ERK1/2 activation occurs within 15min following IR exposure, JNK activation is not observed until 2-3hr post IR (data not shown). Furthermore, JNK inhibition using JNK specific inhibitor SP600125 had little, if any, effect on IRinduced G2/M arrest in MCF-7 cells (data not shown). Furthermore, previous studies have shown that ATM-dependent activation of p38 occurs following irradiation of HeLa and U2OS cells and that this signaling pathway may contribute to IR-induced G2/M arrest in these cells (Wang et al., 2000). However, we observed no increase in p38 phosphorylation following IR exposure of MCF-7 cells (data not shown). Thus, these results indicate that both JNK and p38 are not involved in the regulation of IR-induced G2/M checkpoint activation in MCF-7 cells. Previous studies in H1299 lung cancer cells and HeLa cervical cancer cells have shown that IR exposure induces Cdc25A phosphorylation and that this results in decreased stability of Cdc25A (Xiao et al., 2003; Zhao et al., 2002). While IR treatment of MCF-7 cells was not associated with any concomitant change in the steady state levels of Cdc25A protein (see 1 Figure 1E), ERK1/2 inhibition in MCF-7 cells resulted in increased Cdc25A protein levels even in the absence of IR treatment (see Figure 7C). These results suggest a specific effect of ERK1/2 signaling on the regulation of Cdc25A protein expression and that this effect is independent of ATM/ATR activities, since treatment with caffeine had no effect on Cdc25A protein expression. SUPPLEMENTAL MATERIALS AND METHODS Cell culture and drug treatment For the studies involving drug treatment, log-phase growing cells were incubated in medium containing caffeine (Sigma-Aldrich Corporation), PD98059 (LC Laboratories) or U0126 (LC Laboratories). PD98059 and U0126 were dissolved in DMSO and caffeine dissolved in DMEM. Control cells were incubated in medium containing the same amounts of vehicle alone. For experiments involving IR exposure, exponentially growing cells were treated with IR at the indicated doses and then incubated at 37oC for the indicated times prior to analysis. For experiments involving both drug treatment and IR exposure, cells were pre-incubated with drug for 1hr or 2hr prior to IR exposure. Antibodies and recombinant proteins All antibodies were obtained from Santa Cruz Biotechnology unless indicated elsewhere. These include mouse monoclonal antibodies specific for Cdc2 (17), Cdc25C (H-6), Chk1 (G-4), and Chk2 (B-4); rabbit polyclonal antibodies specific for ATM (Ab-3) (EMD Biosciences), Cdc25A (Cell Signaling Technology), Cdc25C (C-20), Chk1 (FL-476), Chk2 (H-300) and Wee1 (C-20) and goat polyclonal antibody for ATR (N-19). Antibody against phospho-ERK1/2 (E-4) is a mouse monoclonal IgG that binds to a short peptide sequence of ERK1 and ERK2 containing phosphorylated Tyr-204. Antibody C-14-G is a goat polyclonal IgG which recognizes full-length 2 ERK2 and to a lesser extent ERK1. Antibody p-Cdc2 (Tyr15) is a goat polyclonal IgG that recognizes a peptide sequence containing phosphorylated Tyr15 of Cdc2. Anti-phospho-Histone H3 is affinity-purified rabbit polyclonal IgG that recognizes specifically Histone H3 phosphorylated at Ser10 (Upstate Biotechnology). Anti-phospho-p53 antibody (16G8) is a mouse monoclonal IgG1 that detects p53 phosphorylated at Ser-15 (Cell Signaling Technology). As control for protein loading, affinity-purified anti-Actin goat IgG (I-19) was used for quantitating Actin protein levels on all immunoblots. Recombinant Cdc2 protein was purified as a glutathione S-transferase fusion protein containing full-length human Cdc2 (Santa Cruz Biotechnology); Cdc25C protein, substrate for Chk1/Chk2 kinase assay, was purified as a glutathione S-transferase fusion protein containing residues 200-256 of human Cdc25C [kindly provided by Dr. Helen Piwnica-Worms (Washington University School of Medicine)]; p53 full-length protein was purified as a glutathione Stransferase fusion protein (Santa Cruz Biotechnology); Chk1 protein was purified as a glutathione S-transferase fusion protein containing full-length human Chk1 [kindly provided by Dr. Junjie Chen (Mayo Clinic and Foundation)]; glutathione S-transferase protein was used as a negative control substrate in all kinase assays and was prepared according to the standard procedure (Pharmacia); Histone H1 protein was used as a substrate for Cdc2 kinase assay (Upstate biotechnology). Immunoblotting, Immunoprecipitation and kinase assays Cell lysates preparation, immunoblotting and immunoprecipitation assays were performed as described previously (Yan and Mumby, 1999; Yan et al., 2005). For ATM and ATR kinase assays, ATM and ATR proteins were immunoprecipitated from 500g cell lysate using AB-3 (EMD Biosciences) and N-19 (Santa Cruz Biotechnology) respectively. ATM kinase activity was assayed using p53 recombinant protein (Canman et al., 1998; Gatei et al., 2000) and ATR kinase activity assayed using Chk1 recombinant protein (Hall-Jackson et al., 1999; 3 Sarkaria et al., 1999). Both ATM and ATR kinase assays were performed at 30oC for 50min. For Cdc2 kinase assay, Cdc2 protein was immunoprecipitated from 200 g cell lysate using an antiCdc2 antibody (17) (Santa Cruz Biotechnology), and the immunoprecipitates assayed with a Cdc2 kinase assay kit (Upstate Biotechnology) using histone-H1 recombinant protein as substrate. Chk1 and Chk2 were immunoprecipitated from 250g of cell extract, using anti-Chk1 (G4) and anti-Chk2 (B-4) antibody respectively, and kinase assays performed as described previously using Cdc25C recombinant protein as substrate (McGowan and Russell, 1993; McGowan and Russell, 1995; Ward et al., 2001; Yarden et al., 2002; Yu et al., 2002). Chk1 and Chk2 kinase reactions were incubated at 30oC for 20min (Yarden et al., 2002). Wee1 kinase was immunoprecipitated from 500g of cell extract using C-20 anti-Wee1 antibody (Santa Cruz Biotechnology) and Wee1 activity was assayed using Cdc2 recombinant protein as substrate (Santa Cruz Biotechnology) as described previously (Yan et al., 2005). Kinase assays were terminated by addition of 20l of 6 X Laemmli SDS sample buffer and protein substrate phosphorylation analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography (Yan and Mumby, 1999). Substrate phosphorylation of Cdc2 Kinase assay was quantitated either by using a scintillation counter according to manufacture’s instruction (Upstate Biotechnology) or by SDS-PAGE/autoradiography as described previously (Yan and Mumby, 1999). To quantitate protein levels on Western blot, specific protein signals were visualized by chemiluminescence exposed to X-ray film and then scanned using EPSON Perfection 1200XPHOTO scanner. The intensity of individual signals was analyzed using ImageQuant 5.2 analytical program (Amersham Biosciences). siRNA transfection 4 Negative control siRNA, siCONTROL Non-Targeting siRNA (Control-siRNA), contains at least four mismatches to any human, mouse, or rat gene, as previously determined by the manufacture using Microarray. The sequence for the is Control-siRNA 5’- UAAGGCUAUGAAGAGAUAC-3’. SMARTpool siRNAs targeting ERK1/2 consists of eight siRNAs targeting multiple sites on ERK1/2 (ERK1/2-siRNAs). The sequences for ERK1-siRNAs are 5’-PUAAAGGUUAACAUCCGGUCUU-3’, PAGAGACUGUAGGUAGUUUCUU-3’, sequences for ERK2-siRNAs PAGCUUGUAAAGAUCUGUUUUU-3’, 5’-PAACUUGUACAGGUCAGUCUUU-3’, 5’-PUACUGCAACUGCGUGUAGCUU-3’; are and 5’-PAAUAAGUCCAGAGCUUUGGUU-3’, 5’-PUUCUACUUCAAUCCUCUUGUU-3’, 5’the 5’5’- PAAUUUCUGGAGCCCUGUACUU-3’. SMARTpool siRNAs targeting ATR consists of four siRNAs targeting multiple sites on ATR (ATR-siRNAs). The sequences for ATR-siRNAs are 5’PCAAACCAGCAGUGUUGUUCUU-3’, 5’-PUAACAGUAGACAGCUGACCUU-3’, 5’- PUAUCUGUUAGGCGAGUUGCUU-3’ and 5’-PUAAAUAAUCAGCCAUCAGUUU-3’. Cells were transfected with siRNAs at 100nM using DhamaFECT1 siRNA transfection reagent (Dharmacon Research Inc.) according to the manufacture’s instruction. Transfected cells were incubated for additional indicated times and analyzed for protein expression of the relative targeted protein using immunoblotting with specific antibodies. For experiment involving both siRNA transfection and IR exposure, the transfected cells were incubated for the indicated times and then treated with IR. The irradiated cells were incubated for additional indicated times and analyzed for either protein expression or DNA-content as described above. 5 REFERENCES Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K et al (1998). Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281: 16771679. Gatei M, Scott SP, Filippovitch I, Soronika N, Lavin MF, Weber B et al (2000). Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res 60: 3299-3304. Hall-Jackson CA, Cross DA, Morrice N and Smythe C (1999). ATR is a caffeine-sensitive, DNAactivated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene 18: 6707-6713. McGowan CH and Russell P (1993). Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. Embo J 12: 75-85. McGowan CH and Russell P (1995). Cell cycle regulation of human WEE1. Embo J 14: 21662175. Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM et al (1999). Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 59: 43754382. Wang X, McGowan CH, Zhao M, He L, Downey JS, Fearns C et al (2000). Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol Cell Biol 20: 4543-4552. Ward IM, Wu X and Chen J (2001). Threonine 68 of Chk2 is phosphorylated at sites of DNA strand breaks. J Biol Chem 276: 47755-47758. Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S et al (2003). Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem 278: 21767-21773. 6 Yan Y and Mumby MC (1999). Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G(1) cyclin-dependent kinases. J Biol Chem 274: 31917-31924. Yan Y, Spieker RS, Kim M, Stoeger SM and Cowan KH (2005). BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene 24: 3285-3296. Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH and Brody LC (2002). BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet 30: 285-289. Yu Q, La Rose J, Zhang H, Takemura H, Kohn KW and Pommier Y (2002). UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1. Cancer Res 62: 5743-5748. Zhao H, Watkins JL and Piwnica-Worms H (2002). Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A 99: 14795-14800. 7