curriculum vitae - Washington University in St. Louis
advertisement

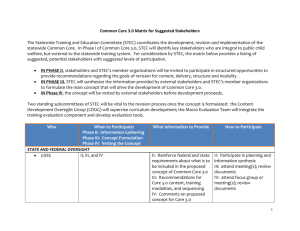
CURRICULUM VITAE Asis Khan Research Associate Department of Molecular Microbiology Washington University in St. Louis Campus Box 8230, 4482-Parkview Place St. Louis, Mo 63110, USA Permanent Address: 134, Nilmoni Bhattacherjee Lane. P.O. Berhampore, Dist- Murshidabad, Pin. 742101 West Bengal India. Present Address: 4482, Lindell Blvd Jackson Arms, Appt# 1006 St. Louis, Mo 63108 USA Personal Column: Nationality: Sex: Marital status: Date of birth Indian Male Single 10th January, 1975 Academic Qualifications: 2003 – Present: Postdoctoral research, Washington University in St. Louis, USA “Constructing a genome map for Toxoplasma gondii” 2003: Ph.D. in Science from Jadavpur University. Title of the Doctoral thesis: Molecular epidemiology and significance of Enterohemorrhagic Escherichia coli O157 (EHEC) and Shiga toxin producing Escherichia coli (STEC) from bovine and human sources. Research Supervisor: Dr. G. Balakrish Nair, Associate Director Laboratory Science Division International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Mohakhali, Dhaka-1212 E. mail: gbnair@icddrb.org 1998: Master of Science, University of Calcutta, India, with Marine Science. 1996: Bachelor of Science, University of Calcutta, India, with Physiology as major subject, Chemistry and Zoology as minor subjects. Fellowship awarded: 1. Junior Research Fellowship from Japan International Co-operation Agency (JICA) project (1998-2000) 2. Senior Research Fellowship from Indian Council of Medical Research (2001-2003) 3. Postdoctoral Research Fellowship from Washington University in St. Louis, USA (2003-2006) Research summary Postdoctoral Research: To provide a framework for linkage mapping and for assembling the scaffolds from the genome sequence, we are presently constructing a 2 cM genetic map of T. gondii. The 10X coverage genome sequence of T. gondii consists of 46 scaffolds that are greater than 100 kb in size (total size ~62 of the estimate ~65 Mb genome). However, the absence of a physical map has hampered the assembly of these scaffolds into chromosomes. The segregation of 142 genetic markers was analyzed among 57 recombinant haploid progeny selected from two different genetic crosses (38 from type I x type III, 19 from type II x type III crosses). Ordered linkage grouping were assembled using MAPMAKER/EXP with a LOD score of 3.0. Chromosome maps were anchored by using specific markers that were mapped previously by karyotype or linkage analysis. The genome scaffolds were assigned to specific linkage groups and ordered by anchoring the genetic markers along the sequence of the underlying contigs. Fourteen distinct linkage groups were identified, corresponding to the previously known 11 chromosomes plus 3 new chromosomes that were previously unapparent due to low coverage. The total genetic map comprises ~604 cM and the average recombination frequency was estimated at ~108 kb per cM. The rate of recombination is lower, and the map unit is correspondingly higher, than that of P. falciparum. In general, the genetic size of the chromosomes was proportional to their physical size with some notable exceptions. For example, chromosome II has a physical size of 2.4 Mb but is < 2 cM, indicating it recombines at a ten-fold lower frequency than average. These linkage maps should prove useful for assembling the whole genome and in identifying genes that regulate drug resistance, virulence, and other biological phenotypes in this important opportunistic human pathogen. Ph. D.: During less than 20 years, strains of Escherichia coli that produce Shiga toxin have emerged as a cause of serious human gastrointestinal diseases and life threatening hemolytic ureamic syndrome. Shiga toxin producing E. coli (STEC) infections have been reported from over 30 countries on six continents, being more common in developed than in developing world. In India, the total death rate of newborn due to diarrhoea alone was estimated to be 10.2% in 1986, but there is a paucity of reports on STEC infections in diarrheal patients as well as in domestic animals. In this context, we have begun present study as initial step towards understanding the prevalence of STEC infections in Calcutta and to get a better insight into the predominant virulence marker, pathogenic potential of these isolates in causing diarrhoea and mode of transmission. The isolation rate of STEC among hospitalized secretory and bloody diarrhea cases was very low being 0.8% and 2.5% respectively, although the prevalence of STEC was high in bovine fecal flora (19.6%) and in beef (50%). PCR tests indicated that 36.1%, 20.8% and 43.0% STEC strains harbored stx1, stx2 and both stx1 and stx2, respectively, while eae was present in only 13.9% of the strains. E-hlyA was the most prevalent (30.6%) plasmid encoded marker as compared to katP (12.5%) and etpD (4.2%). Comparison of the virulence gene profiles of Calcutta STEC strains isolated from different sources, demonstrated the relative abundance of katP gene in cow and beef isolates (12.2% and 25%, respectively) as compared to human strains (11.1%). Likewise, there was at least two times higher incidence in the percentage of hlyA PCR positive STEC of bovine origin (39.0%) than those isolated from human (18.5%). 30 STEC strains were serotyped, of which 8 were O antigen untypable with somatic (O) antisera. Of human isolates 4 new STEC serotypes (O96:H19, ONT:NM, ONT:H18 and ONT:H14) were identified. No clustering of any particular serotype could be seen among the isolates. Approximately 49.2% strains showed antibiotic resistance to one or more of the antimicrobial agents used in this study. Resistance was observed most commonly to ampicillin, tetracycline and streptomycin, and less frequently to cephalothin, co-trimoxazole, nalidixic acid and neomycin. 97% STEC were cytotoxic to Vero cells while 44.6% showed a hemolytic phenotype that resembled -hemolytic activity rather than typical entohemolytic phenotype. 60% of strains from human, 80% of cow and all the isolates from beef samples were sorbitol fermenter, fermentation occurred within 24 hours. STEC strains from diverse sources were analyzed by RAPD-PCR and PFGE to assess their genetic relatedness. Except few strains the overall RAPD-PCR and PFGE banding pattern showed high degree of heterogeneity. The clonal diversity among STEC indicates less possibility of transmission of strains from food chain to human. While searching for E. coli O157 in aquatic environment, 5 strains of Citrobacter braakii were fortuitously detected, which cross-reacted with the commercially available O157 polyvalent antiserum using an immunodetection procedure. The LPS profile of these strains differed from that of the O157:H7 E. coli strain and a clinical isolate of Citrobacter spp. although they reacted with the monoclonal O157 antibody. Hybridization with DNA probes generated from O157 O-antigen biosynthesis gene cluster indicated that apart from weak reactions with one or two of the DNA probes, all the C. braakii strains did not yield positive signals suggesting their lack of resemblance to E. coli O157 apart from structural mimicry. In conclusion, this survey showed that STEC strains could not be implicated as major causal agent of diarrhea but are present in the food chain in Calcutta. What exactly will trigger such strains to potentiate outbreaks remains to be seen. Given that STEC are present in the food chain in Calcutta and given that STEC are not important human enteropthogen at the present time, it would be useful to trace the natural history of the organism, should they become important enteropathogens in the not too distant future. Other scientific activity 1. 2. 3. 4. Japan International Cooperation agency (JICA)- National Institute of Cholera & Enteric diseases (NICED) training programmed on “Molecular Epidemiology of Diarrhoeal Diseases with Special Reference to Cholera” held at NICED, Calcutta, November 27th- December 6th, 2000. JICA- NICED training programmed on “Molecular Epidemiology of Diarrhoeal Diseases with Special Reference to Cholera” held at NICED, Calcutta, November 1st- November 10th, 2001. JICA- NICED training programmed on “Molecular Epidemiology of Diarrhoeal Diseases with Special Reference to Cholera” held at NICED, Calcutta, August 12stAugust 22th, 2002 Received a training on isolation, induction, screening of Stx phage from environmental samples as well as from clinical strains of Shigella dysentery type I under the supervision of Dr. S. M. Faruque in International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Mohakhali, Dhaka-1212 from 11 November to 11December, 2001. Research skill Microbiology: a) b) c) Isolation, identification, and maintenance of pure cultures of various bacterial strains (including members of the families Enterobacteriaceae (specially Shiga toxin producing Escherichia coli and Vibrionaceae) from clinical and environmental sources. Serotyping of clinical and environmental isolates of Shiga toxin producing Escherichia coli. Development of drug sensitivity pattern and determination of minimum inhibitory concentration for various pathogens. Molecular Biology: a) b) c) d) e) f) g) h) i) j) k) l) m) n) o) p) Constructing a genome map Preparation and purification of chromosomal DNA. Preparation and purification of plasmid DNA. Detection of genes employing polymerase chain reaction. Preparation and labeling of non-radioactive probe. Colony hybridization assay using non-radioactive DIG labeled probe. Dot Blot assay Randomly amplified polymorphic DNA. Pulsed Field Gel Electrophoresis (PFGE). Isolation of bacteriophage DNA. Isolation of phages from environment. Cloning/sub-cloning into plasmid vectors Sequencing of the various amplified PCR product. Genetic analysis by restriction fragment length polymorphism (RFLP). Southern hybridization using non-radioactive probes. Identification of various organisms through 16S-rDNA sequencing. Immunology: a) b) c) d) e) f) g) h) i) j) k) Detection of various bacterial toxins by bead ELISA Detection of various bacterial toxins by plates ELISA. Dot ELISA Preparation of Lipopolysaccharide (LPS). Preparation of outer membrane protein. Protein analysis by SDS-PAGE gel electrophoresis by Comassie blue staining. LPS analysis by SDS-PAGE gel electrophoresis by silver nitrate staining. Immunoblot assay. Bactericidal assay of human serum sample. Detection of bacterial hemolysin by blood agar plate method. Contact hemolysin assay. Tissue Culture: a) b) c) Revival, maintenance, subculture and preservation of T. gondii Revival, maintenance and preservation of different cell lines, e.g., HeLa, CHO, Vero etc. Bacterial adherence and toxin study using Vero, HeLa and CHO cell lines. Animal Experiment: a) b) c) Virulence study of T. gondii in mice Toxin assay by suckling mice Effect of different toxins on Using chamber experiment. LISTS OF PUBLICATIONS: 1. 2. 3. 4. Basu A, Garg P, Datta S, Chakraborty S, Bhattacharya T, Khan A, Ramamurthy S, Bhattacharya SK, Yamasaki S, Takeda Y, Nair GB. A seven-year analysis of the incidence, antibiogram and genotypes of Vibrio cholerae O139 in Calcutta, India and comparison of the Calcutta O139 genotypes with those isolated from other parts of India. Emerg. Infect Dis. 2000; 6(2): 139-147. Khan A, Yamasaki S, Sato T, Ramamurthy T, Pal A, Datta S, Chowdhury NR, Das SC, Sikdar A, Tsukamoto T, Bhattacharya SK, Takeda Y, Nair GB. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga Toxin-Producing Escherichia coil isolated from cattle, beef and human cases in Calcutta, India. Emerg. Infect Dis. 2002; 8(1): 54-62. Khan A, Nandi RK, Das SC, Ramamurthy T, Khanam J, Shimizu T, Yamasaki S, Bhattacharya SK, Chicumpa W, Takeda Y, Nair GB. Environmental isolates of Citrobacter braakii that agglutinate with Escherichia coil O157 antiserum but do not possess the genes responsible for the biosynthesis of O157 somatic antigen. Epidemiol. Infect. 2002; 129: 1-8. Khan A, Das SC, Ramamurthy T, Sikdar A, Khanam J, Yamasaki S, Takeda Y, G. Balakrish Nair. Antibiotic resistance, virulence genes and molecular profiles of Shiga Toxin-Producing Escherichia coil isolated from diverse sources in Calcutta, India. J Clin Microbiol. 2002; 40(6): 2009-2015. 5. 6. 7. 8. 9. 10. 11. Sinha S, Chakraborty R, De K, Khan A, Datta S, Ramamurthy T, Bhattacharya SK, Takeda Y, Nair GB. Escalating association of Vibrio cholerae O139 with cholera outbreaks in India. J Clin Microbiol. 2002; 40(7): 2635-7. Chakraborty S, Khan A, Kahali S, Faruque SM, Yamasaki S, Ramamurthy T. Infantile diarrhoea associated with sorbitol-fermenting, non-shiga toxin-producing Escherichia coli O157:H-. Eur J Clin Microbiol Infect Dis. 2003; 22(5):324-6. Epub 2003 May 08. Datta S, Khan A, Nandy RK, Rehman M, Sinha S, Chattopadhyay S, Das SC, Nair GB. Environmental isolates of Aeromonas spp. harboring the cagA-like gene of Helicobacter pylori. Appl Environ Microbiol. 2003; 69(7): 4291-5. Das SC, Khan A, Panja P, Datta S, Sikdar A, Yamasaki S, Takeda Y, Bhattacharya SK, Ramamurthy T and Nair GB. Tracking the origin and circulation of Shiga toxinproducing Escherichia coli (STEC) in a dairy farm in Kolkata, India using molecular traits. (Communicated) Datta S, Chattopadhyay S, Nair GB, Mukhopadhyay AK, Hembram J, Berg DE, Saha DR, Khan A, Satra A, Bhattacharya SK and Chowdhury A. Virulence genes and neutral DNA markers of Helicobacter pylori isolates from different ethnic communities of West Bengal, India. J Clin Microbiol. 2003; 41(8): 3737-43. Pandey M, Khan A, Das SC, Sarkar B, Kahali S, Chakraborty S, Chattopadhyay S, Nandy RK, Bhattacharya SK, Yamasaki S, Takeda Y, Nair GB and Ramamurthy T. Association of cytolethal distending toxin locus cdtB with enteropathogenic Escherichia coli isolated from acute diarrheal patients in Calcutta, India. J Clin Microbiol. 2003; 41(11): 5277-81. Khan A, Datta S, Das SC, Ramamurthy T, Khanam J, Takeda Y, Bhattacharya SK, Nair GB. Shiga toxin producing Escherichia coli infection: current progress & future challenges. Indian J Med Res. 2003; 18:1-24 Review. Published abstracts of papers presented at different scientific meeting: 1) 2) 3) 4) Khan A, Pal A, Das SC, Sikdar A, Ramamurthy T, Tsukamoto T, Yamasaki S, Sato T, Takeda Y, Nair GB. Prevalence and genetic profiling of virulence traits of Shiga toxin-producing Escherichia coli isolated in Calcutta, India. 4th International Symposium and Workshop on "Shiga Toxin (Verocytotoxin) -Producing Escherichia coli Infections" Kyoto, Japan, Oct 29 -2 Nov 2000. Khan A, Pal A, Das SC, Sikdar A, Ramamurthy T, Tsukamoto T, Yamasaki S, Sato T, Takeda Y, Nair GB. Prevalence and genetic profiling of virulence traits of Shiga toxin-producing Escherichia coli isolated in Calcutta, India. 5th International Meeting on " Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases" Hyderabad, India.Nov 12-16, 2000. Das SC, Khan A, Ramamurthy T, Yamasaki S, Sikdar A, Bhattacharya SK, Nair GB and Takeda Y. Identification and characterization of Shiga toxin-producing Escherichia coli in hospitalized acute diarrhoea cases and in cattle in Kolkata, India. 9th Asian Conference on diarrheal diseases and nutrition. New Delhi, India. 28-30 Sep, 2001. Khan A, Das SC, Ramamurthy T, Yamasaki S, Takeda Y and Nair GB. A comparison of phenotypes, virulence genotype and clonality of STEC strains isolated from clinical cases of diarrhea, cattle feces and marketed beef in Kolkata, India. X th International Congress of Bacteriology and Applied Microbiology, Paris, 27th July to 1st August, 2002. 5) Khan A, Su C, Cole R, Tang K, Glover D, Gajria B, Li Mackey LA, Fraunholz M, Roos D, Berriman M, Paulsen I, Ajioka J, and Sibley LD. Constructing a genome map for Toxoplasma gondii. Host Parasite Interaction, Gordon Research Conferences, University of Rhode Island, West Kingston, RI 02892-0984, USA, 27th Jun to 1st July, 2004. References 1. Dr. G. Balakrish Nair, Deputy Director, National Institute of Cholera and Enteric Diseases. P-33, C.I.T. Road Scheme XM. Calcutta- 700010. INDIA. Present Address : Associate Director Laboratory Science Division International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Mohakhali, Dhaka-1212 E. mail: gbnair@icddrb.org 2. Dr. L. David Sibley Professor Department of Molecular Microbiology Washington University in St. Louis Campus Box 8230, 4482-Parkview Place St. Louis, Mo 63110, USA E. mail: sibley@borcim.wustl.edu 3. Dr. T. Ramamurthy Assistant Director, National Institute of Cholera and Enteric Diseases. P-33, C.I.T. Road Scheme XM. Calcutta- 700010. INDIA. E. mail: trama@yahoo.com