Balancing Redox Reactions Oxidation Number Method
advertisement
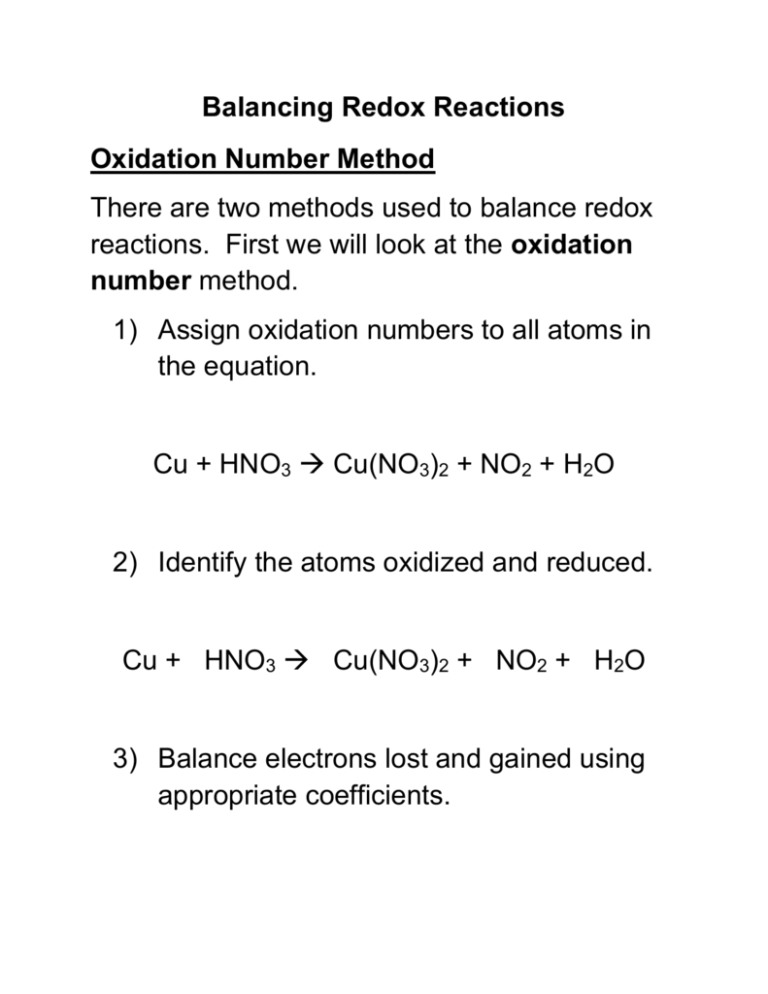
Balancing Redox Reactions Oxidation Number Method There are two methods used to balance redox reactions. First we will look at the oxidation number method. 1) Assign oxidation numbers to all atoms in the equation. Cu + HNO3 Cu(NO3)2 + NO2 + H2O 2) Identify the atoms oxidized and reduced. Cu + HNO3 Cu(NO3)2 + NO2 + H2O 3) Balance electrons lost and gained using appropriate coefficients. Cu + HNO3 Cu(NO3)2 + NO2 + H2O 4) Make the change in oxidation numbers equal in magnitude by adjusting coefficients in the equation. Cu + HNO3 Cu(NO3)2 + NO2 + H2O 5) If necessary, use the conventional method to balance the remainder of the equation. Cu + HNO3 Cu(NO3)2 + NO2 + H2O Try this one yourself: K2Cr2O7 + H2O + S → SO2 + KOH + Cr2O3 Balancing Redox Reactions Oxidation Number Method Balance the following aqueous oxidation reduction reaction that occurs in an acidic solution. MnO4– + I–→ MnO2 + I2 Step 1: Assign oxidation numbers to all the atoms in the reaction. Write the number above the appropriate atoms and show electrons lost and gained. Step 2: Determine the change in oxidation number and what is being oxidized and reduced. Step 3: Make the change in oxidation numbers equal in magnitude by adjusting coefficients in the equation. Step 4: Add up the ion charges and balance with H+ since the reaction occurs in an acidic solution. Step 5: Water is now added to the opposite side to balance the H and O atoms. Try: ClO3- + Cl- Cl2 + ClO2 Balancing Redox Reactions Balance the following aqueous oxidation reduction reaction that occurs in a basic solution. MnO4– + C2O42– → CO2 + MnO2 For basic solutions, instead of balancing with H+(aq), use OH-(aq). Then add water to the opposite side to balance the O and H atoms. Try: Zn + NO3- ZnO22- + NH3











