Cycle 3 Natural World Study Guide
advertisement

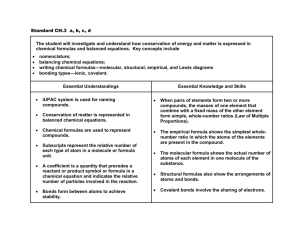
NAME POWER: NATURAL WORLD CYCLE 3, 2016 Overview The Greek philosopher Democritus first suggested the idea that matter is made of tiny indivisible particles, and called them atoms. In the late 18th century, a modern theory about atoms originated. Many chemical reactions were studied and the weights of substances involved were carefully measured. John Dalton’s atomic theory arose from these observations. He believed that the atoms of an element were identical and different from those of any other element. Two or more of these atoms could join together in chemical combination producing “molecules” of substance called compounds. A pure compound contains a single kind of molecule. Chemical bonds are formed using the electrons in the outer shell of the atoms. Atoms will lose, gain, or share their electrons in the process. _____1. Read the overview with the class. Complete the vocabulary words. _____2. Advanced: See teacher for details. Guiding Question1: What are the ways in which elements bond together to make chemical compounds and how are those compounds named? ____3. Participate in the lesson on bonding and different types of bonds. Take notes. ____4. Participate in the lesson on Lewis dot structures, ions and neutral compounds. Take notes. ____5. Participate in the lesson on chemical formulas and nomenclature. Take notes. ____6. IW: Read and highlight “Introduction to Covalent Molecules Model.” ____7. GW: Complete the following activities. a. The “Covalent Molecule Worksheet” activity with the molecular bonding set. Complete the “Table Worksheet.” b. The “Ion Chips” activity sheet. ____8. IW: Complete the following worksheets: a. “Chemical Symbols and Formulas” b. “Great Combinations” c. “Nomenclature & Chemical Formulas” Guiding Question 2: What are the types of chemical reactions and how are chemical equations balanced? During chemical reactions, the total mass of the reactants equals the total mass of the products. This principle is called the Law of Conservation of Mass. The description of a chemical reaction that occurs is often summarized using a shorthand method called a chemical equation. A balanced equation represents the law of conservation of mass by telling how many atoms of each element react to form one or more new substances. Because chemical equations follow the law of conservation of mass, the number of atoms of each element must be the same on both NAME POWER: NATURAL WORLD CYCLE 3, 2016 sides of the arrow. Chemists say that such an equation is balanced. For example, carbon reacts with oxygen to form Carbon Dioxide in the following reaction: C + O2 CO2 To check if the equation is balanced, check the atoms on both sides of the equation. On the reactant side (left), there is one carbon atom and two oxygen atoms. On the product side (right), there is also one carbon atom and two oxygen atoms. Not all chemical equations are automatically balanced. Sometimes, numerical coefficients are used in order to balance an equation, as in the equation below: 4H2 + 2O2 4H2O ____9. Participate in the lesson on types of chemical reactions, catalysts, and the “energy” of reactions. Take notes. ____10. Participate in the lesson on balancing chemical equations. Take notes. ____11. Complete the following worksheets. a. “A Voyage through Equations” section 1 and 2. b. “Balancing Equations Race” ____12. GW: Complete the following experiments: a. “Temperature and Reaction Speed” b. “Cabbage Juice Indicator” ____13. IW: Complete a full complete lab report on “Temperature and Reaction Speed.” How do I put it all together? ____14. Project: In your small group, create a children’s book about one of the topics below. The text for the book must be typed, and the pictures hand-drawn. a. Ionic Bonding e. Chemical Equations b. Covalent Bonding f. Acids and Bases c. Metallic Bonding g. Types of Reactions d. Hydrogen Bonding