Title of the Paper (16pt Times New Roman, Bold, Centered)
advertisement

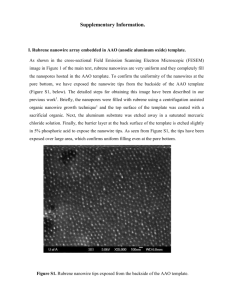
The effect of etching parameters for removal of AAO template as Cu2O/Cu nanowires fabricated by electrochemical deposition Y. H. Leea, I. C. Leub, M. T. Wua, J. H. Yena, M. H. Hon aand K. Z. Funga* a Department of Materials Science and Engineering National Cheng Kung University b Department of Electronic Engineering Kun Shan University of Technology a No.1 Ta-Hsueh road, Tainan 701 Taiwan Abstract: -This article reports our recent results of electrochemically deposited Cu 2O/Cu nanowires by AAO template assisted method. The deposited Cu2O/Cu nanowires were observed by SEM and TEM under different removal condition. The results indicated that the composition and surface morphologies strongly affected by the condition as the removal of AAO template. We find that the well-controlled condition of AAO removal is a key point to maintain the nano-structure. The optimum condition for AAO removal is decided as pH = 12 of NaOH solution. Key-Words: - Cu2O, Cu, Electrochemical deposition, Nano-wires, AAO template 1 Introduction Recently, the nano-sized materials have received much attention due to their excellent physical and chemical properties. The electrochemical deposition was regarded as an inexpensive and easy method to obtain nano-structure without post heat-treatment and vacuum. The AAO ( Anodized Aluminum Oxide ) template assisted method is a newly developed nano-fabrication technique for aligned nano-arrays. Our group has reported several results of the combination of electrochemical deposition and AAO template assisted method【1-4】. Cu2O and its related material has been noticed for its particular optical and electrical properties【5-7】. Furthermore, Cu2O has also been reported to be a promising anode material for lithium ion battery【8-10】. We successfully synthesized Cu2O/Cu composite nanowires by electrochemical deposition combined with the assistance of AAO template. After the removal of AAO template by NaOH, it was found that the morphologies of nanowires were pH dependent. 2 Experimental procedure 2.1 AAO template preparation The high purity Au and Al films were deposited on to Si wafer by E-beam evaporation. The anodization of Al was first performed in a bath consisting of 0.3M oxalic acid solution under 40V at 13℃. After removing the oxide in a mixture of 12wt% H3PO4 and 6wt% H2Cr2O4, the remaining Al film was again oxidized till it was all converted to alumina. The samples were then immersed into 5wt% H3PO4 solution in order to remove the barrier layer in the Al/Au interface. The evaporated Au layer was used as a conductive layer for the electrochemical deposition. 2.2 Deposition parameters The Cu2O/Cu nanowires were synthesized from an aqueous solution prepared from 0.6M Cu(Ⅱ)and 3M lactate that contained copper ( Ⅱ ) sulfate pentahydrate ( Showa ) and lactic acid (Riedel-deHaën). After adding certain amount of 5M NaOH, the solution was then stirred overnight with a magnetic stirplate. The stabled solution was then adjusted to desired pH 10. The electrochemical deposition system employed is a conventional three-electrode cell. An AAO mounted by epoxy was used as working electrode. The counter electrode is Pt foil while an Ag/AgCl was used as reference electrode in a 3M NaCl solution. The deposition was performed with an potentiostat/galvanostat (EG&G, Model 273A) under a constant current density. The current density was set at 0.05 mA/cm2. The deposition was terminated when the channels of AAO were filled. The deposited specimen was then cleaned with deionized water to remove the remaining impurities. 2.3 The removal of AAO template The deposited samples were immersed in NaOH solution to remove AAO template. The pH of NaOH varied from 11.0 to 13.5. The duration of immersion was determined to be 30 minutes. After immersion, the specimens were rinsed by deionized water to clean remaining NaOH. 2.3 The morphological observation and structural analysis The porous alumina substrate and deposited nanowires were then observed by Scanning Electron Microscope(Philips, XL-40FEG) and Transmission Electron Microscope (JEOL, JEM-3010). The structural analysis was conducted using TEM and glancing angle x-ray diffractometer ( D-Max, Rigaku). 3 Results and discussion Shown in Fig.1 is the top-view SEM observation of an AAO template. From the micrograph, the well-aligned porous alumina were fabricated onto the Au coated Si substrate. The pore diameter is about 60 nm and length is 300 nm. The density of pores is approximate 1*1011 per cm2. The ratio of exposed pore to overall exposed area is about 0.33. The AAO template was then immerse in the Cu-containing lactate solution. Subsequently, the electrochemical deposition was conducted on the AAO template. The Cu and Cu2O were electrochemically deposited into the pore of AAO template. The Cu2O/Cu filled AAO was then immerse in NaOH solution with varied pH values. Fig. 2(a) shows the surface of deposited sample after etched with NaOH solution (pH=11.0). This figure indicated that AAO still remained intact and was not etched by NaOH solution. When the pH of NaOH solution increased to 11.5, AAO template was partially removed as shown in Fig. 2(b). At the same time, the Cu2O/Cu nanowires were exposed. As the pH increased to 12, all of AAO template was removed by NaOH solution. Thus, well-aligned Cu2O/Cu nanowires with diameter of 60nm and length of 300nm were clearly observed in Fig. 2(c). When the pH of NaOH solution increased to higher values as shown in Fig.2 (d), the removal of deposited Cu2O/Cu was locally seen. Therefore, the deposited Cu2O/Cu nanowires was not stable in the very basic solution. The deposited naowires were analyzed by a glancing angle XRD. The scanning rate was set at 4°/min from 20 ° to 80 ° of 2 θ angles. The result of diffraction was plotted in Fig.3. The reflected peak has been identified as the (110) (111) (200) (220) reflective planes of Cu2O and (111) (200) (220) of Cu. The reflection marked with the star symbol is from the substrate. This result shows that the nanowires consisted of Cu2O and Cu composite were fabricated by combined electrcochemical deposition and AAO template assisted methods. The deposited nanaowires were also observed by TEM observation. The bright-field image is shown in Fig. 4. The diameter of nanowires is in a good agreement with the observation by SEM. Furthermore, the image also shows the polycrystalline of Cu2O/Cu composite. The well-defined diffraction rings (not shown here)also indicated the several responsible reflection planes of Cu2O and Cu. 4 Conclusion Based on these results, Cu2O/Cu nanowires were successfully fabricated using AAO-assisted electrochemical deposition method. However, the pH value of NaOH etching solution plays an important role in the fabrication of Cu2O/Cu nanowires. When the pH of NaOH etching solution was less than 12, Cu2O/ Cu nanowires were not completely exposed due to the partially dissolution of AAO template. Under pH=12, AAO template was entirely removed without the dissolution of Cu2O/Cu nanowires. The well-aligned Cu2O/Cu nanowires with the diameter of 60nm and length of 300nm were obtained. Acknowledgement The authors gratefully acknowledge the financial support by National Science Council of Taiwan. (Grant No. NSC 92-2120-M-006-003) References: [1]Y. C. Wang, I. C. Leu. M. H. Hon, “Effect of colloid characteristics on the fabrication of ZnO nanowires arrays by electrophoretic deposition”, J. Mater. Chem., Vol.12, 2002, p.2439-2444 [2]Y. C. Wang, I. C. Leu. M. H. Hon, “Preparation of nanosized ZnO arrays by Electrophoretic deposition”, Electrochem. Solid-state Lett., Vol.5, No.4, 2002, p.C53-C55 [3]S. T. Chang, I. C. Leu, M. H. Hon, “Preparation and characterization of nanostructured tin oxide films by electrochemical deposition”, Electrochem. Solid-state Lett., Vol.5, No8, 2002, C71-C74 [4] M. T. Wu, I. C. Leu and M. H. Hon, “Effect of polishing pretreatment on the fabrication of ordered nanopore arrays on aluminum foils by anodization”, J. Vac. Sci. Technol. B, Vol.20 No. 3, 2002, p.776 [5] J. Lee, and Y. Tak, “Electrochemical Deposition of a Single Phase of Pure Cu2O Films by Current Modulation Methods”, Electrochem. Solid-State Lett., Vol.3, No.2, 2000, p.69-72 [6] T. D. Golden, M. G. Shumsky, Y. Zhou, R. A. VanderWerf, R. A. Van Leeuwen. and J. A. Switzer ,“Electrochemical deposition of copper(Ⅰ) oxide films”, Chem. Mater., Vol.8, 1996, p.2499-2504 [7] P. E. de Jongh, D. Vanmaekelbergh, and J. J. Kelly,Chem. Mater., “Cu2O :electrochemical deposition and characterization”, Vol.11, 1999, p. 3512-3517 [8] P. Poizot, S. Laruelle, S. Grugeon, L. Dupont, and J. M. Tarascon, “Nano-sized transition–metal oxides as negative electrode materials for lithium ion batteries”, Nature, Vol.407, 2000, p.496-499 [9] B. Laik, P. Poizot, and J. M. Tarascon, “The electrochemical quartz crystal microbalance as a mean for studying the reactivity of Cu2O toward lithium”, J. Electrochem. Soc., Vol.149, No.3, 2002, p.A251-A255 [10] S. Grugeon, S. Laruelle, R. Herrera-Urbina, L. Dupont, P. Poizot, and J. M. Tarascon, “Particles size effect on the electrochemical performance of copper oxides toward lithium”, J. Electrochem. Soc., Vol.148, No.4, 2001, p.A285-A292 1.2μm Fig.1 The top-view SEM observation of porous AAO template 514nm Fig.2 (a) The morphology of deposited nanowires after etched in NaOH solution (pH=11.0) 2.25μm Fig.2 (b) The morphology after etched in NaOH solution (pH=11.5) 1.8μm Fig.2 (c) The morphology after etched in NaOH solution (pH=12.0) Fig.4 The bright-field image of deposited Cu2O/Cu nanowires 1.8μm (220) (200) 20 30 40 50 2 Fig.3 The XRD pattern of deposited nanowires (220) (200) Relative intensity Cu2O (110) Cu (111) (111) Fig.2 (d) The morphology after etched in NaOH solution (pH=12.5) 60 70 80