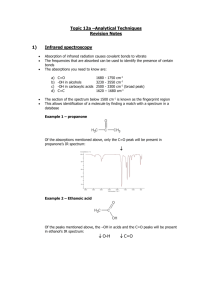
Infrared Spectrometry
advertisement

Infrared Spectrometry Section 7I, Chapters 16 & 17 Remember the energy diagram of a molecule as previously discussed. The energy of infrared radiation corresponds with the vibrational (as opposed to electronic for UV-visible radiation) energy levels of molecules. Molecular vibrations are basically dependent on two parameters: 1. The masses of the atoms involved in the vibration 2. The strength of the bond holding the atoms together. Thus infrared spectrometry provides a lot of structural/functional group information (as opposed to electronic UV-visible spectrometry) in molecules. Even though there is totally different information content between the two techniques the spectrum, in a fundamental sense, is generated the same way. 1 Historically the instrumentation of UV-Vis and IR spectrometers were very similar. They were often both single channel, usually double beam instruments. Now single channel infrared spectrometers are extinct, and multichannel infrared spectrometers have not yet been perfected. Fourier transform infrared spectrometers rule this region of the electromagnetic spectrum – single beam, single channel, multiplexing. The UV/Vis instruments discussed thus far work in the frequency domain. That is, the measurements are made as a function of frequency (or wavelength). Fourier transform (FT) spectrometers work in the time domain. That is, measurements are made as a function of time, then a Fourier transform (an advanced mathematical manipulation to the data) is done to convert that to a frequency domain spectrum. 2 The basics of optical FT spectroscopy first introduced in Section 7I. (Note that FT spectroscopy also dominates NMR instrumentation, and is also a major player in MS instrumentation (FT-MS, ICR). The principles are the same, the details of how the time domain information is generated differs.) Time domain and frequency domain spectra have the same information content. As you know, for some historical reasons the x-axis in infrared spectrometry is in units of cm-1. The mid-IR region where fundamental molecular vibrations occur is from 4000 – 200 cm-1. This corresponds to frequencies of 1.2 x 1014 – 6.0 x 1012 s-1. You can conceive of acquiring a time domain spectrum with the following instrumental configuration. 3 Detectors do not respond to power variations at these high frequencies, they have a finite “rise time”. The rise time for a PMT or vacuum phototube is 10-8 – 10-9 s. The rise time for a typical IR detector is 10-3 – 10-4 s. Since the detector rise time is << the frequency of radiation, the time domain output from such an instrument would contain no frequency information. The high frequency signal must be modulated to a low frequency signal that available detectors can respond to. The most common way to do this is to use a Michelson interferometer. 4 1. Parallel beam strikes beamsplitter, half of light transmitted, half reflected. 2. One beam reflects of fixed mirror, 2nd off moving mirror. 3. Beam recombines at beamsplitter, half is reflected to detector. If you put a line source (1 frequency) through the interferometer, the output is determined by the path difference between the beams when traveling to fixed and movable mirrors. Retardation δ = 2(OM – OF) 1. When OM = OF, δ = 0. Beams in phase when recombined at the beamsplitter, constructive interference, maximum detector signal. 2. When OM and OF differ by 0.25λ, δ = 0.5. So pathlength difference is 0.5λ. Beams out of phase when recombined at the beamsplitter, destructive interference, minimum detector signal. The detector signal is a cos function with max signal when δ = nλ 5 If you know the distance δ between crests in cos function [I(δ)], get λ (ν) of monochromatic radiation in interferometer. The amplitude of the cos function is proportional to signal intensity. If you move the mirror at a constant known velocity, and measure the time for constructive interference to occur [I(t)], you will also know I(δ), and can get I(λ) and I(ν). I(t) = time domain spectrum I(ν) = frequency domain spectrum The time domain spectrum has been modulated to a lower frequency as a result of passing through the interferometer, the frequency information in the time domain signal can now be detected. I(t) = ∫ I(ν) cos2πtdν This cos Fourier transform finds the frequencies and intensities which fit the time domain spectrum using a computer algorithm (FFT using Cooley-Tukey algorithm). For a continuous source (used in FTIR), all waves constructively interfere when δ = 0. The signal decays rapidly after that. 6 Note that an FTIR has no monochromator, no slits, so much more source intensity reaching detector. For methods which are detector noise limited (IR, because the detectors aren’t so great), this is very important for high S/N. (Throughput or Jacquinot Advantage). As with a multichannel instrument, these multiplexing FT instruments afford very fast spectral acquisition times compared to single channel instruments. Thus signal averaging to enhance S/N is practical. 7 A small sampling from Chapter 16, 17. Near-IR region: 13,000 cm-1 4000 cm-1 (800 nm 2500 nm) Used a lot for quantitative analysis of industrial processes. Instrumentation more like UV-Vis Mid-IR region: 4000 cm-1 400 cm-1 Historically used for organic qualitative analysis. Now that and more. Energy: 500 nm = 2.4 kJ/mol. 5000 nm (2000 cm-1) = 0.24 kJ/mol Absorbed energy results in a change in molecule’s dipole moment, so the amplitude of molecular vibration increases. What dictates the vibrational frequency? Start with Hooke’s Law. At the atomic scale 8 For single bonds k ~ 500 N/m For double bonds k ~ 1000 N/m For triple bonds k ~ 1500 N/m Calculate the approximate cm-1 of absorption for a C=O stretching vibration. μ = 1.1 x 10-26 kg Calculate the approximate cm-1 of absorption for a C-O stretching vibration. 9 This is the origin of “group frequencies” for functional group qualitative analysis. C=O, C=C, C-H, O-H etc. all have absorption frequencies dictated by bond strength and reduced mass. FTIR is also useful for quantitative analyses. Beer’s Law applies for a transmission measurement whether it be in the UV-Vis or IR region. The enhanced selectivity of IR over UV-Vis is the single most important difference between these instrumental methods. Transmission techniques for obtaining IR spectra of gases, liquids, solids. Window materials require more thought than for UV-Vis. Gases: easy with the right cell. Allow the gas to expand, long pathlengths often required because of low concentration. 10 Liquids (solutions). Solvent absorption a problem, spectral subtraction of the solvent can be a successful approach. For quantitative work a reproducible (and short) pathlength is required. Determining the exact pathlength can be done by obtaining a “spectrum” of the empty sample holder. 11 Reflected radiation from cell walls interferes with transmitted radiation producing interference pattern. Constructive interference occurs when reflected radiation has traveled distance equal to an integer multiple of the wavelength of the nonreflected transmitted radiation. λ = 2b/N Solids. Particle size must be < radiation λ to avoid scattering. Can do one of 2 things to acquire transmission spectra: KBr pellet – about 1 mg of sample in 100 mg KBr. Grind, mix, press to 15,000 psi. Yields a transparent disk when done correctly. Mulls – make dispersion of finely ground sample ~2 mg in a mineral oil or fluorinated hydrocarbon. Problem – interference with dispersant. Reflectance IR Spectroscopy Mirrored solid surface by specular reflectance where angle of incidence = angle of reflection 12 Powdered material instead of mull or KBr pellet 13 Spectra of other solids or liquids that can make intimate contact with an internal reflection element can be acquired by ATR – Attenuated Total Reflectance. This utilizes the same optical phenomenon used for optical fibers (Chapter 7 Section G) Chapter 16 questions/problems: 1, 3, 8, 10, 13 Chapter 17: 10 14