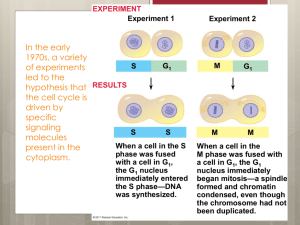
DNA-damage Inducible Transcript3-p53`s potential partner in crime
advertisement

DNA-damage Inducible Transcript 3 - a new member in p53’s cell death club Shweta Bhawalkar Each living cell on earth stores its hereditary information in the form of DNA. A gene is a segment of DNA sequence that encodes a protein. Mutations - changes in the DNA sequence - in such segment can result in cancer. Thus, cancer is in part a genetic disease. It is now an established fact that more than half of the human cancers are caused by a mutation in the gene encoding protein p53. Since its discovery 25 years ago, p53 has changed the chapters in the tumor biology field. The protein p53 forms the major cellular hub that recruits a vast array of proteins for triggering activities such as introducing ‘brake’ in the cell growth cycle and causing cell death or cell suicide, also known as apoptosis. p53 is also known to be involved in cellular aging, copying cellular DNA, specialization of cells, development, and repair of damage to the DNA. In normal cells, the level of p53 is kept low. DNA-damaging agents such as ultraviolet and gamma radiation, carcinogens (substances causing cancer), and drugs having toxic effect on cells cause considerable damage to the DNA. When the p53 protein senses this damage its expression is upregulated, leading to its accumulation in the nucleus and either a pause in cell growth (cellcycle arrest) or apoptosis, depending on the extent of damage. How p53 senses the damage and how it decides whether the cell should undergo cell cycle arrest or apoptosis are still unknown. The p53 responds diferently to cellular stress in different tissues and under different conditions. Computational methods have identified more than 4000 possible p53 binding sites in the human genome. Previous work in this laboratory has demonstrated one binding site for p53 in the DNAdamage inducible transcript 3 (DDIT3) gene, and that this gene is upregulated to make more protein when the oxygen level is low (hypoxia). DDIT3 is induced in response to DNA damage and is implicated in growth arrest and apoptosis. Therefore, I wanted to determine whether p53 takes the help of DDIT3 to induce apoptosis in response to ionizing radiation. Unlike for hypoxia, my results show that DDIT3 is expressed independently of p53, and the level of induction was the same irrespective of the radiation dosage. Thus, the question arises as to how p53 upregulates the DDIT3 in hypoxia as well the role of DDIT3 in hypoxia-induced apoptosis. Further experiments should help in understanding and obtaining a clearer picture of the p53 function in apoptosis. Degree project in biology, University of Uppsala, autumn/spring 2005/2006 Examensarbete i biologi, 20 p Biology Education Centre, Uppsala University and Cancer Center Karolinska, Karolinska Hospital, Solna Supervisor: Prof. Klas G. Wiman & Tao Liu