Biology (National 5) - Disease transmission practical
advertisement

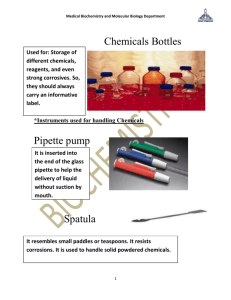
DISEASE TRANSMISSION PRACTICAL Disease transmission practical This practical work illustrates for learners the importance of taking careful notes in scientific investigation. Each learner must take individual notes at the time to be able to successfully complete the exercise. Several versions of this experiment are provided for use as appropriate with your learners. Introduction Some people are carriers of disease. These carriers show either no symptoms associated with that disease or only mild symptoms. At some point they may eventually become ill. The danger to others is that a carrier may not be recognised as having the disease. Carriers have the pathogen inside them, however, and may spread it to people they come into contact with. In this way carriers may spread HIV hepatitis, and a number of other diseases. It is one of the reasons HIV spreads so quickly. For some diseases, public health officials must identify the original carrier. This person is sometimes referred to as the ‘Typhoid Mary’. By identifying such a person and finding out who he/she came in contact with, officials learn how the disease is being passed from person to person. In this activity, one of you will be the original carrier of a ‘disease’. (This disease is perfectly harmless, if handled properly. You will carry it in a testtube, rather than in your body.) The original carrier will make contact with several learners in the class who will then make contact with others. Then all learners will be tested to see who has become infected. In this activity you will trace the path by which a disease spreads, using a pipette to transfer solutions from one test -tube to another, and testing a solution for the presence of an acid or a base. Materials and equipment needed Pasteur or disposable pipette with bulb, 3 ml Test-tubes with stoppers/corks, 14–18 ml Phenol red 0.001 mol/l hydrochloric acid (HCl, stock solution of non-carriers) 0.1 mol/l sodium hydroxide (NaOH, stock solution of the carrier) Safety goggles Lab coat ADVICE AND GUIDANCE FOR PRACTITIONERS (NATIONAL 5, BIOLOGY) © Crown copyright 2012 1 DISEASE TRANSMISSION PRACTICAL Gloves Marker pen/pencil Pre-activity discussion Acids and bases are very often colourless solutions. Concentrated acids and bases can result in serious burns, especially to exposed membranes such as those of the eyes. They can damage clothing and furniture. Acids and bases must, therefore, be properly labelled. The acids and bases you use in this activity are relatively dilute and pose little harm unless they get in your eyes or mouth. The concentration of an acid or base is indicated by a number called the molarity (mol/l). Look at the materials and equipment list. Which is greater in molarity, the acid (HCl) or the base (NaOH)? Phenol red is a pH indicator. It changes colour depending upon whether the solution it is added to is an acid or a base. If phenol red is added to an acid, the solution will be yellow. If it is added to a base, the solution will be red. Safety precautions Do not allow acids and bases to come into contact with your skin or clothing. Wear gloves, goggles and a lab coat through the entire procedure. Ensure that you do not transfer spills or drips from gloves to other surfaces such as pens where you may transfer contamination to your hands and mouth. Method 1. 2. 3. 4. 5. 6. 2 Collect your sample of unknown solution and , using a Pasteur or disposable pipette, transfer three pipettes of your unknown solution to a clean sample tube. Make sure you label your tube so it doesn’t get mixed up with another sample and keep your original sample secure. You may need to use it for checking later on. Choose someone at random from the group to be your cont act in the first round. Remove one pipette of solution from your sample tube. Empty your pipette into your contact’s sample tube at the same time as he/she does the same to you. Replace the lid on your sample tube and shake your tube gently to mix the solutions. Record the name of the person with whom you exchanged solutions in the Round 1 contacts column. ADVICE AND GUIDANCE FOR PRACTITIONERS (NATIONAL 5, BIOLOGY) © Crown copyright 2012 DISEASE TRANSMISSION PRACTICAL 7. When everyone has completed Round 1, repeat steps 2 –5, recording the name of the contact in the Round 2 column. Make sure you meet a new contact each time by choosing someone from a different table or group. 8. Once everyone has completed Round 2, repeat steps 2 –5 for Round 3. 9. When you have exchanged solutions with three different contacts, it is time to think about the test. (a) (b) (c) Is the disease curable? Some diseases may have no cure as yet, e.g. HIV. What are the consequences of being found positive? Do you feel you need more information or sup port in the form of counselling? When ready proceed to step 10. 10. Add one drop of phenol red to your test -tube to see if you are infected with the disease. Two test results are possible: Solution turns red – you have the disease. What are the implications? Solution turns yellow – you are not infected. 11. Record your result in the table. Indicate a red with a positive (+), and yellow with a negative (–). Learner name Test result Round 1 Contacts Round 2 ADVICE AND GUIDANCE FOR PRACTITIONERS (NATIONAL 5, BIOLOGY) © Crown copyright 2012 Round 3 3 DISEASE TRANSMISSION PRACTICAL Collate data You should now collate the class data (names, contacts and test results) into the data table provided. Trace the transmission of the disease to each learner who tested positive. Can you determine who the original carrier was? Carrier: ………………………………………………………………………….. Result of confirmation test: ………………………………………… ……….… (Adapted from Applied Biology/Chemistry, Unit 7 ‘Disease and Wellness’ (1990), The Centre for Occupational Research and Development, Waco, Texas.) 4 ADVICE AND GUIDANCE FOR PRACTITIONERS (NATIONAL 5, BIOLOGY) © Crown copyright 2012 DISEASE TRANSMISSION PRACTICAL Data table Learner name Test result Round 1 Contacts Round 2 Round 3 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. ADVICE AND GUIDANCE FOR PRACTITIONERS (NATIONAL 5, BIOLOGY) © Crown copyright 2012 5