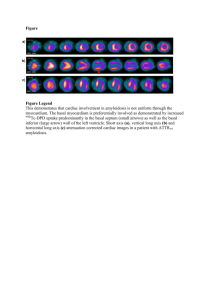
Case Report:
advertisement

Case Report: Hyperkalemia in a patient with familial Mediterranean fever and amyloidosis Tamara L Hoppe1,2 Aviva Y Rostas1,2 Nicole B Sitzer1,2 1 Michael G. DeGroote School of Medicine, McMaster University University of the Negev, Be’er Sheva, Israel 2 Ben-Gurion nicole.sitzer@medportal.ca ABSTRACT Familial Mediterranean Fever is a hereditary periodic fever syndrome primarily affecting Mediterranean populations and manifesting with recurrent attacks of fever and serositis. As with many chronic inflammatory states, amyloidosis is a serious complication. Colchicine is used to manage FMF by reducing inflammation and preventing secondary amyloidosis, but has several undesired effects. We present a patient with FMF complicated by amyloidosis and renal failure. Through this case presentation, we will discuss chronic inflammation as an underlying cause of systemic amyloidosis. KEYWORDS: Familial Mediterranean Fever, Amyloidosis, Colchicine, Periodic Fever Syndrome HISTORY Patient X is a 62 year-old Jewish male of Iraqi descent, with a history of FMF and consequent amyloidosis, chronic renal failure (baseline creatinine 155 mmol/L), chronic anemia, and hypertension. As well, he has multiple sclerosis and gout. He has a strong family history of FMF and was diagnosed in childhood. Patient X described experiencing recurrent attacks of severe abdominal and leg pain, as well as swelling in the calves. Although his current medications included colchicine, this treatment was not available at the time of disease onset. Unfortunately, due to this extended period of uncontrolled inflammation, he developed secondary renal amyloidosis and chronic renal failure. Prior to hospitalization, Patient X presented to his family physician with a onemonth history of diarrhea, fatigue, and weakness. Bloodwork was unremarkable, with the exception of a potassium (K+) of 6.2 mmol/L (3.5-5). The patient was sent to the emergency department (ED) and admitted to the internal medicine service. PHYSICAL EXAMINATION AND INVESTIGATIONS Upon presentation to the ED, his vital signs were within normal limits. Both physical examination and chest X-ray were unremarkable. Bloodwork revealed an elevated creatinine of 195 mmol/L, urea of 9.8 mmol/L (3.0-6.5), K++ of 5.7 and hemoglobin of 98 g/L (130-180). Additionally, his creatine kinase (CK) was elevated, but the exact value was unknown. Of note, EKG findings were abnormal revealing peaked T waves on the chest leads consistent with potentially arrhythmogenic hyperkalemia. The patient was urgently treated with calcium gluconate, ventolin, and kayexalate. FAMILIAL MEDITERRANEAN FEVER Familial Mediterranean Fever (FMF) is an autosomal recessive disease, characterized by recurrent paroxysmal attacks of fever (>40C) and serositis, including peritonitis and pleuritisi. The presentation often resembles an acute surgical abdomen, with onset frequently between 10-20 years of ageii. FMF is most prevalent in inhabitants of the Mediterranean basin including Sephardic Jewish, Arabic, Turkish, and Armenian populationsiii. A long-term, serious complication of FMF is secondary amyloidosisiv, which damages multiple organ systems including the kidneys, gastrointestinal (GI) tract, liver, spleen, heart, thyroid and testesv. It is important to have an understanding of this disease because Canada’s population is multicultural and immigrant-rich, and cases of FMF may present in local hospitals. Additionally, FMF is an archetypal hereditary periodic fever syndrome and shares complications and treatment principles with other diseases of this type such as TNF receptor-1 periodic fever syndrome and Hyper IgD syndrome. FMF is caused by a mutation, typically M694V in the MEFV gene on chromosome 16p, which results in the production of a defective Pyrin (marenostrin) protein. Normally, Pyrin is expressed on circulating neutrophils and acts to decrease the systemic inflammatory responsevi. However, in cases of FMF, the mutant Pyrin protein fails to blunt the inflammatory response, producing widespread neutrophil-dominant inflammationvii. The current standard treatment is colchicine, a plant extract, made available in 1972. Colchicine binds a drug domain on microtubules inhibiting neutrophil motility and blunting the inflammatory responseviii. It is absorbed in the distal small bowel and the primary route of excretion is via the hepatobiliary system ix. Side effects include GI upset, neutropenia, and myopathyx. This report characterizes the types of amyloidosis as well as its clinical manifestations. In the case presented, we also discuss the pathophysiology of amyloidosis, renal failure, and other sequelae of FMF. AMYLOIDOSIS Amyloidosis includes a collection of diseases that are characterized by abnormal protein-folding. The atypical proteins often assemble into toxic and insoluble pleated sheets with resultant systemic or organ-specific protein deposition. The two most common subtypes of amyloidosis are of the systemic nature, and they include light chain amyloidosis (AL) and reactive amyloidosis (AA) xi. Light chain amyloidosis involves deposition of monoclonal Ig light chain, and occurs primarily in multiple myeloma, monoclonal gammopathy of undetermined significance, and Waldenström’s macroglobulinemiaxii. Reactive amyloidosis is distinguished by deposition of serum amyloid A protein. It occurs as a consequence of chronic inflammatory states such as chronic infection, and various inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease and periodic fever syndromesxiii. Based on the pervasiveness of these etiologies, reactive amyloidosis is expected to be more prevalent. Chronic inflammation perpetuates the release of cytokines, particularly interleukin-1, from activated macrophages. These cytokines stimulate increased hepatocyte production of acute phase reactants, including serum amyloid A, which is broken into smaller fragments (AA) by circulating monocytes and macrophagesxiv. These fragments then deposit into various tissues, resulting in tissue damagexv. Complications include heart failure, GI pathology, bowel perforation, and renal diseasexvi. With respect to renal amyloidosis, protein deposition may be glomerular or vascular, both impairing filtration resulting in chronic renal failurexvii. The diagnosis of possible amyloidosis is based on history and clinical presentation and is then verified by a renal or rectal tissue biopsy. Under polarized light, amyloid proteins, if present, bind to Congo red staining and will show pathognomonic apple-green birefringencexviii. We present a case encountered at Soroka Hospital in Be’er Sheva, Israel, a country in the Mediterranean with a large population susceptible to FMF. The patient described typifies the natural history, management, and complications of FMF and amyloidosis. ANALYZING THE INVESTIGATIONS There are multiple possible etiologies for the acute presentation of hyperkalemia in Patient X. He had diarrhea, likely as an adverse effect of his colchicine treatment or as a GI manifestation of amyloidosis. The diarrhea likely led to a volume-depleted state, exacerbating his chronic renal failure, and resulting in hyperkalemia. Furthermore, his elevated CK, consistent with myoglobin toxicity as an adverse effect of colchicine treatment, may have led to acute tubular necrosis due to the toxicity of myoglobin to renal tubular cells. It was suspected that the patient’s presentation may have been aggravated by a diet rich in potassium. CONCLUSION This report used FMF as an example to illustrate the causal relationship between chronic inflammation and amyloidosis. The knowledge gained from this case may be applied to a host of other inflammatory states including malignancies and various rheumatological conditions. The etiologies of amyloidosis are common, and it is important to aggressively identify and treat chronic inflammation to mitigate the occurrence of this preventable and devastating complication. ACKNOWLEDGMENTS We the authors thank Dr. Mahmud Abu-Shakra of Ben-Gurion University of the Negev for his encouragement and guidance throughout our elective. REFERENCES i Lidar M, Kedem R, Berkun Y, Berkun Y, Langevitz P, Livneh A. Familial Mediterranean Fever in Ashkenazi Jews: the mild end of the clinical spectrum. J Rheumatol 2010 Dec:37(2) 422-25. ii Sohar E, Gafni J, et al. Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med 1967 43: 227-53. iii Bakkaloglu A. Familial mediterranean fever. Pediatr Nephrol 2003 18: 853-59. iv Lidar M, Kedem R, Berkun Y, Berkun Y, Langevitz P, Livneh A. Familial Mediterranean Fever in Ashkenazi Jews: the mild end of the clinical spectrum. J Rheumatol 2010 Dec:37(2) 422-25. v Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003 Aug;349:583-96 vi Drenth JPH, van der Meer JWM. Hereditary periodic fever. New Engl J Med 2001 Dec:345:1748-57. vii Ben-Chitrit E, Levy M. Familial Mediterranean fever. Lancet. 1998 May 30;351(9116)1658-9. viii Stanton RA, Gernert KM, Nettles JH, Anjea R. Drugs that target microtubules: A new molecular perspective. Med Res Rev. 2011 March doi:10.1002/med.20242. ix Cocco G, Chu DCC, Pandolfi S. Colchicine in clinical medicine. Eur J Intern Med. 2010: 21Dec:21(6):503-8. x Drenth JPH, van der Meer JWM. Hereditary periodic fever. New Engl J Med 2001 Dec:345:1748-57. xi Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003 Aug;349:583-96. xii Robert, AK, Therneal, TM, Rajkumar, SV, Offord JR, Larson DR, Larson MS, Plevak MR, Melton LJ. A long term study of prognosis in monoclonal gammopathy of undetermined significance. New Engl J Med. Feb;346:564-9. xiii Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003 Aug;349:583-96. xiv Glenner, GG. Amyloid deposits and amyloidosis. New Engl J Med 1980 June;302:1283-92. xv Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003 Aug;349:583-96. xvi Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003 Aug;349:583-96. xvii Glenner, GG. Amyloid deposits and amyloidosis. New Engl J Med 1980 June;302:1283-92. xviii Glenner, GG. Amyloid deposits and amyloidosis. New Engl J Med 1980 June;302:1283-92. [1] Petitti, DB, Crooks VC, Buckwalter JG, Chiu V. Blood pressure levels before dementia. Arch Neurol. 2005 Jan;62(1):112-6. Author Biographies: Tamara Hoppe, Aviva Rostas, and Nicole Sitzer are second year medical students at McMaster University. Prior to medical school, they studied in the Bachelor of Sciences program at the University of Western Ontario. This past summer, they traveled to Be’er Sheva, Israel, for an elective in International Health and Internal Medicine at Soroka Hospital associated with Ben Gurion University of the Negev. While abroad, they observed interesting presentations of diseases commonly encountered in Mediterranean populations and hope to continue to travel and work together in the future.