06-Amyloidosis for the ladies
advertisement

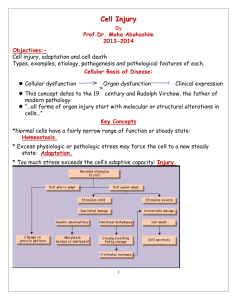
Amyloidosis for the young ladies Amyloidosis Definition : In medicine, amyloidosis refers to a variety of conditions in which amyloid proteins are abnormally deposited in organs causing disease. A protein is amyloid if, due to an alteration in its secondary structure, it takes on a particular insoluble form, called the beta-pleated sheet. AMYLOIDOSIS • Disease characterized by extracellular deposition of pathologic insoluble fibrillar proteins in organs and tissues. • Term amyloid first coined by Virchow in mid 19th century (meaning starch or cellulose). • Amyloid found to stain with congo red, appearing red microscopically in normal light but apple green when viewed in polarized light. • Fibrillar nature and beta pleated sheet configuration described by electron microscopy in 1959. Amyloidoses Are Protein Misfolding Diseases Binding sites for Congo red Protein Misfolding Diseases A specific protein may be unable to carry out its normal function because it is improperly folded or because it is not sufficiently stable due to misfolding. A protein may be unable to carry out its normal function because it is not trafficked to the proper location due to misfolding. A protein may fail to fold or to remain folded correctly and consequently aggregate (often with other components) leading to amyloid diseases. (“Amyloidosis” refers strictly to diseases in which extracellular deposits are formed, but the terms “amyloid diseases” or “protein aggregation diseases” are now being used to refer to diseases in which protein deposits are intra- or extracellular.) Some of the clinical symptoms of the non-neurological amyloidoses seem to be due to the accumulation of large deposits of aggregated proteins in vital organs In neurodegenerative diseases, cellular function appears to be impaired by the interaction of aggregated proteins with cellular components. This impairment is associate with evidence of elevated oxidative stress, but the mechanism is unknown. Pathogenesis of the major forms of amyloid fibrils Systemic amyloidoses are those which affect more than one body organ or system. Localised amyloidoses affect only one body organ or tissue type. Primary amyloidoses arise from a disease with disordered immune cell function such as multiple myeloma and other immunocyte dyscrasias. Secondary (reactive) amyloidoses are those occurring as a complication of some other chronic inflammatory or tissue destructive disease. • Imaging techniques – Technetium Tc 99m pyrophosphate binds avidly to many types of amyloid. Quantitative assessment not possible and strongly positive results usually only occur in pt’s with severe disease. Technetium labeled aprotinin may be more sensitive. • Quantitative scintigraphy can be done with iodine-123- labeled serum amyloid P component (sensitive for AL, ATTR and AA amyloid). Multiple Myeloma: Incidence and Etiology • 13,000 cases/year in USA • Median age - 65 yrs. • Incidence in African-Americans is two-fold other ethnic groups • Familiar clusters are rare • Environmental/occupational exposures have been implicated AL AMYLOIDOSIS • Part of the spectrum of plasma cell dyscrasias. • Cellular source of AL amyloid is always a single clone of the B-lymphocytic lineage, usually exhibiting the morphologic appearance of plasma cells. • Underlying clonal proliferative disorder may be frankly neoplastic (ie:multiple myeloma) or conversely a low grade proliferation of monoclonal plasma cells. Macroglossia hoarsenes Heart failure Autonomic nervous system involvement Carpal tunnel sy Peripheral nervous system involvement Thrombocytosis AL AMYLOID Splenomegaly Anemia Hypofunction of adrenal glands hypothyreosis Hepatomegaly Nephrotic syndrome • Among MM patients, amyloidosis reported with variable frequency, but rarely exceeds 20%. • Majority of patients without myeloma associated AL, occurs in the setting of an apparently “benign” monoclonal gammopathy. • Characterized by low concentrations of monoclonal Ig’s in serum/urine and an often occult low grade monoclonal plasma cell proliferation in BM. Bone marrow aspirate: Plasma cell infiltrate AL Amyloidosis IF anti l Kidney (74%) Heart (58%) Bone marrow plasma cell clone synthesizing amyloidogenic light chain No of organs involved: 1 (31%) >1 (69%) Liver (28%) GI (8%) Primary Amyloidosis: Histopathology Conditions Associated With AA Amyloidosis Chronic Inflammatory Diseases Rheumatoid arthritis Psoriatic arthritis Chronic juvenile arthritis Ankylosing spondylitis Behcet’s syndrome Reiter’s syndrome Adult Still's disease Chronic Infections Tuberculosis Osteomyelitis Bronchiectasis Leprosy Pyelonephritis Decubitus ulcers Whipple’s disease Acne conglobata Inflammatory bowel disease Hereditary periodic fevers Common variable immunodeficiency Hypo/agammaglobulinemia Cystic fibrosis Neoplasia Hepatoma Renal carcinoma Castleman's disease Hodgkin's disease Adult hairy cell leukemia Waldenström's disease Presenting Clinical Features in AA Amyloidosis Feature % Proteinuria/renal insufficiency 91 Diarrhea/malabsorption 22 Goiter 9 Neuropathy/carpal tunnel syndrome 3 Hepatomegaly 5 Lymphadenopathy 2 Cardiac 1 -2 • Macroglossia – occurs in 10-20 % • Amyloid can be found within any part of the GI tact and may infiltrate parenchyma, organs and nerves. • Peripheral neuropathy may be presenting manifestation or develop subsequently during the course of the illness (history of carpal tunnel frequently elicited). • Neuropathy usually distal, symmetric and progressive. Cranial nerve and autonomic nerve involvement also well described. • Motor neuropathy rare. Macroglossia Purpura HEPATIC/SPLENIC • Involvement of liver common. • Hepatomegaly may be striking at presentation and usually disproportionate to extent of liver enzyme abnormalities (except alkaline phosphatase which is frequently elevated). • Presence of jaundice is an adverse prognostic factor and MST from onset of jaundice is only 3 months. • Patients may present with severe intrahepatic cholestasis. • Massive splenic deposition may result in functional hyposplenism. Extensive hypertrophy with yellow amyloid deposits CARDIAC • May present with rapid and progressive onset of CHF. • Characteristically, features are predominantly of right sided CHF. • ECG – low voltage and may have a pattern of MI in absence of CAD. • ECHO – concentrically thickened ventricles with normal-small cavity and diastolic dysfunction on doppler. • Clinical clue is marked worsening of failure when CCB used. Echocardiogram revealing thickened walls with small chambers RENAL • Nephrotic syndrome present in 30-50% at diagnosis. • Nephrotic syndrome and renal failure develop only rarely during course of the illness if not present at time of diagnosis. • λ BJP have been associated with inferior survival as compared with κBJP or no monoclonal protein, irrespective of serum creatinine. Lambda Kappa • AL arthropathy – may simulate RA. Most striking appearance is the ‘shoulder pad sign’ secondary to swelling of the shoulder joints. • Vascular infiltration may result in easy bruising especially in the eyelids and flexural regions. Purpuric lesions typically occur above the nipple. • Factor X deficiency (acquired) can occur in up to 10% of pt’s and over 2/3 of pt’s with acquired factor X deficiency have systemic Amyloidosis. Amyloidosis Heart This islet of Langerhans demonstrates pink hyalinization (with deposition of amyloid) in many of the islet cells. This change is common in the islets of patients with type II diabetes mellitus. PROGNOSIS • Serious disease with high mortality. • Overall median survival after diagnosis is < 2years in most series. • Patients with co-existent MM have a poorer prognosis. • Survival time largely dependent upon the organ system predominantly involved. • Cardiac involvement is major determinant of prognosis and most common cause of death – MST from onset of CHF is 7 months. • Results of a a large trial of 220 pts by Kyle et al in 1997 clarified the role of colchicine in AL Amyloidosis. • Median duration of survival was 8months for the C group, 18 months for the MP group and 17 months for the MPC group. • Median survival for pts with cardiac amyloid was 5 months, 16 months for pts with renal involvement and 34 months for those with PN. Survival was best in those patients showing a 50% reduction in serum or urine paraprotein levels. CONCLUSIONS • Systemic, uncommon disease with poor long term survival. • Symptoms often vague and recognition of syndromes associated with amyloidosis is key. • In general, current therapy is suboptimal although new treatment options including thalidomide, proteosome inhibitors, antisense oligonucleotides and SCT hold promise for the future.