1. Consider the reaction H2(g)
advertisement
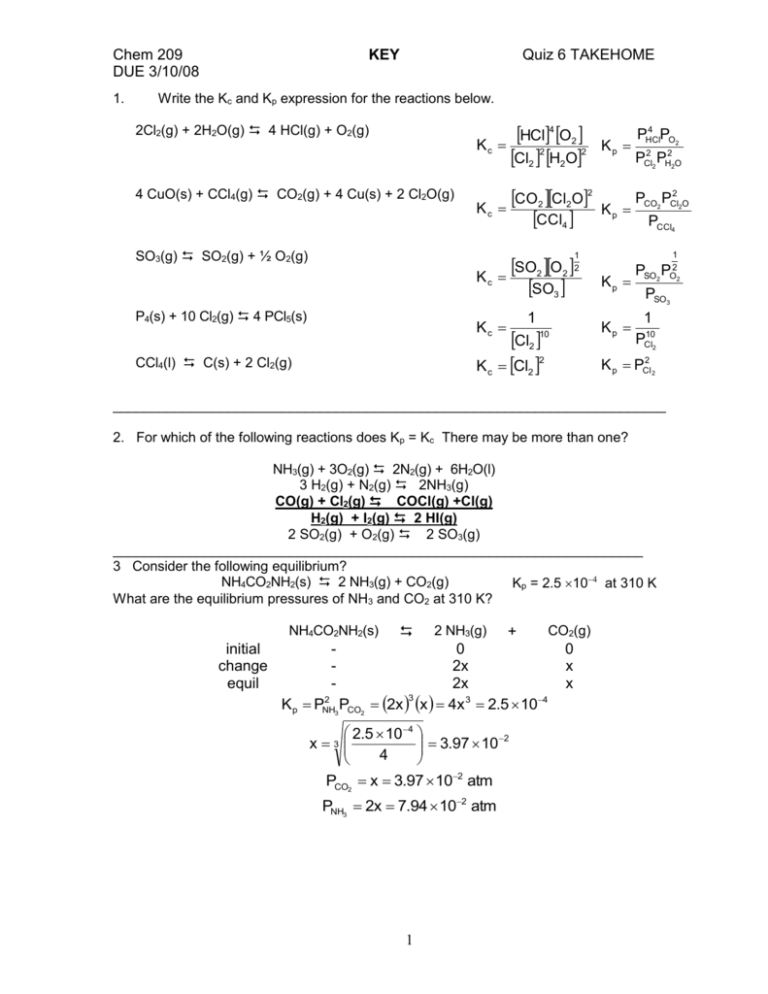
Chem 209 DUE 3/10/08 1. KEY Quiz 6 TAKEHOME Write the Kc and Kp expression for the reactions below. 2Cl2(g) + 2H2O(g) 4 HCl(g) + O2(g) 4 CuO(s) + CCl4(g) CO2(g) + 4 Cu(s) + 2 Cl2O(g) SO3(g) SO2(g) + ½ O2(g) Kc HCl4 O2 Cl2 2 H2O2 Kp Kc CO2 Cl2O2 CCl4 Kp SO2 O2 2 Kc SO3 4 PHCl PO2 PCl2 2 PH22O PCO2 PCl2 2O PCCl4 1 1 P4(s) + 10 Cl2(g) 4 PCl5(s) 1 Kc Kp Cl2 2 K c Cl2 CCl4(l) C(s) + 2 Cl2(g) Kp 10 PSO2 PO22 PSO3 1 PCl102 K p PCl2 2 ________________________________________________________________________ 2. For which of the following reactions does Kp = Kc There may be more than one? NH3(g) + 3O2(g) 2N2(g) + 6H2O(l) 3 H2(g) + N2(g) 2NH3(g) CO(g) + Cl2(g) COCl(g) +Cl(g) H2(g) + I2(g) 2 HI(g) 2 SO2(g) + O2(g) 2 SO3(g) _____________________________________________________________________ 3 Consider the following equilibrium? NH4CO2NH2(s) 2 NH3(g) + CO2(g) Kp = 2.5 10 at 310 K What are the equilibrium pressures of NH3 and CO2 at 310 K? NH4CO2NH2(s) initial change equil 2 K p PNH P 3 CO2 2 NH3(g) + 0 2x 2x 3 2x x 4x 3 2.5 104 2.5 104 3.97 102 x 3 4 PCO2 x 3.97 102 atm PNH3 2x 7.94 102 atm 1 CO2(g) 0 x x Chem 209 DUE 3/10/08 KEY Quiz 6 TAKEHOME ___________________________________________________ 4. Consider the exothermic reaction 4 NO2(g) + O2(g) 2 N2O5(g). Indicate the effect of the following stresses on the equilibrium. (shift to products, shift to reactants, no effect) a) a decrease in the pressure of NO2 (shifts to reactants) b) an increase in the pressure of N2O5 (shifts to reactants) c) a decrease in temperature. (shift to products) d) addition of a catalyst (no effect) e) an increase in the volume of the container holding the gases. (shifts to reactants) ______________________________________________________________________ 5. Consider the reaction 4 CuO(s) + CCl4(g) Kp at 400 2(g) + 4 Cu(s) + 2 Cl2O(g). °C is 625 for this reaction. Write the algebraic equation needed to solve for the equilibrium pressures of CCl4(g), CO2(g), and Cl2O(g) at 400 °C. Assume initially that the concentrations are 1.0 atm for CCl4(g) , 2.0 atm for CO2(g) and 3.0 atm for Cl2O(g). 4 CuO(s) 4 Cu(s) + 2 Cl2O(g) + CCl4(g) CO2(g) + initial change equil - 1.0 x 1.0 - x Kp PCl2 2OPCO2 PCCl4 2.0 x 2.0 - x - 3.0 2x 3.0 - 2x 2 3.0 2x 2.0 x 625 1.0 x _______________________________________________________________________ 6. For the reaction 2 SO2(g) + O2(g) 2 SO3(g), Kc = 0.200 at 100 °C. If 1.0 moles of SO2 is mixed with 0.50 moles of SO3 and 0.50 moles of O2 in a 5.0 L chamber, will there be more or less than 1.0 mole of SO2 when equilibrium is reached. (Hint: use the reaction quotient to determine the answer) SO3 0.50 mol 0.10 M 5L SO2 1.0 mol 0.20 M O2 0.50 mol 0.10 M 5L Q 5L SO3 2 0.12 2.5 SO2 2 O2 0.22 0.1 since Q > K there are too many products and thus will be more SO2(s) at equilibrium _______________________________________________________________________ 7. Find the concentration of Mg2+ ions in a saturated solution of MgF2 (Ksp = 6.6x10-9) MgF2(s) i c e Pb2+ 0 x x + 2F 0 2x 2x K sp Mg2 F 1 2 x(2x)2 4x 3 1 K 3 6.6 10 9 3 1.2 10 3 x sp 4 4 2+ [Mg ] = x = 1.210 2 Chem 209 DUE 3/10/08 KEY Quiz 6 TAKEHOME __________________________________________________________________________ 8. Assume a reaction has a H of 55.0 kJ/mol. It's equilibrium constant at 25 oC is 6.410-2. Find the equilibrium constant at 35 oC. K H 1 1 ln 35 K 25 R T35 T25 55000Jmol 1 1 K 35 1 1 1 K 25 8.314JK mol 308K 298K K ln 35 6615K 3.247 10 3 K 1 3.356 10 3 K 1 K 25 ln ln ln e K 35 6615K 1.090 10 4 K 1 0.72 K 25 K 35 K 25 e 0.72 K 35 2.056 K 25 K 35 k 25 2.056 6.4x10 2 0.132 3






![CHEM 1520 SI MON, TUES, & WEDNES 1.Calculate [H3O+] in a](http://s3.studylib.net/store/data/007346334_1-b78d73402f58153c92290299886ff084-300x300.png)


