Supplementary Information
advertisement

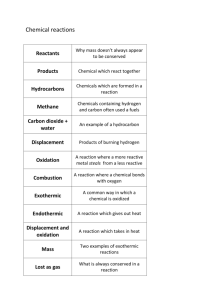
-1- Supplementary Information In-Situ TEM visualization of vacancy injection and chemical partition during oxidation of Ni-Cr nanoparticles Chong-Min Wang1*, Arda Genc2, Huikai Cheng2, Lee Pullan2, Donald R. Baer1, and Stephen M. Bruemmer3 1 Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA 2 FEI Company, 5350 NE Dawson Creek Drive, Hillsboro, Oregon 97124, USA 3 Fundamental and Computational Science Directorate, Pacific Northwest National Laboratory, Richland, WA 99352, USA This supplementary information includes the following contents: 1: Ni5Cr-S1.avi: Captured in-situ TEM movie shows the structural evolution of a single Ni5Cr nanoparticle oxidized at 375 °C in a gas mixture of 25% O2 and 75% Ar with a total pressure of 1 mbar in the ETEM column around the sample. Note the injection of vacancies, nucleation of the cavity, and growth of cavity 2: Ni5Cr-S2.avi: Captured in-situ TEM movie shows the structural evolution of a cluster of Ni5Cr nanoparticles oxidized at 375 °C in a gas mixture of 25% O2 and 75% Ar with a total pressure of 1 mbar in the ETEM column around the sample. 3: Ni-S3.avi: Captured in-situ TEM movie shows the structural evolution of a cluster of Ni nanoparticle oxidized at 375 °C in a gas mixture of 25% O2 and 75% Ar with a total pressure of 1 mbar in the ETEM column around the sample. Fig. S1: STEM-HAADF image and EDS maps showing the structure and elemental distribution in the Ni5Cr particle that is exposed to air at room temperature. The EDS reveals that following the initial oxidation at room temperature, the oxide layer is rich in Cr, indicating a preferential oxidation of the Cr even at the initial oxidation stage. Fig. S2: STEM-HAADF image and EDS maps showing the structure and elemental distribution in the Ni5Cr particle that is oxidized in the ETEM column at 375 °C in a gas mixture of 25% O2 * electronic mail: chongmin.wang@pnnl.gov -2and 75% Ar with a total pressure of 1 mbar. The EDS reveals that the Cr-rich oxide layer is trapped by the NiO formed during high temperature oxidation. Fig. S1: STEM-HAADF image and EDS maps showing the structure and elemental distribution in the Ni5Cr particle that is exposed to air at room temperature. The EDS reveals that following the initial oxidation at room temperature, the oxide layer is rich in Cr, indicating a preferential oxidation of the Cr even at the initial oxidation stage. -3- Fig. S2: STEM-HAADF image and EDS maps showing the structure and elemental distribution in the Ni5Cr particle that is oxidized in the ETEM column at 375 °C in a gas mixture of 25% O2 and 75% Ar with a total pressure of 1 mbar. The EDS reveals that the Cr-rich oxide layer is trapped by the NiO formed during high temperature oxidation.