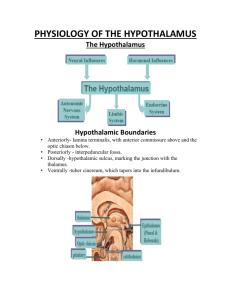
In situ hybridization

Supplementary Materials
In situ hybridization. Brains were removed immediately after decapitation, frozen in a bed of crushed dry ice, sectioned in a coronal plane at 14 µm with a cryostat, mounted on
RNase-free slides, and treated with 4% paraformaldehyde, acetic anhydride, ethanol and chloroform
1
. For each animal, 6 slides (12 brain sections) containing hypothalamus were selected from the midregion of the rostro-caudal extent of the arcuate nucleus (which also contains the ventromedial nuclei). The anatomical equivalence of hypothalamic sections among animals was obtained by selecting slides (viewed with a darkfield stereomicroscope) with the aid of a rat brain atlas prior to hybridization. All brain slices were concurrently prepared for hybridization and used in the same assay. Hybridization to LRb mRNA was performed with an antisense riboprobe transcribed from a cDNA template using (
33
P) UTP
2
, and unincorporated label was separated using a QIAquick
Nucleotide Removal Kit (Qiagen). The hybridization signal was detected using a phosphoimager system (Packard).
Stat3 Nuclear Translocation . The protocol was modified from Hubschle et al, 3 . After leptin treatment, mice were anesthetized with Avertin and transcardially perfused with saline followed by 10% buffered formalin at room temperature. Brains were postfixed for two hours in the same fixative and cryoprotected in 20% sucrose in PBS overnight at 4 o C.
Serial 40
m coronal sections were cut using a sliding microtome. Free-floating sections were rinsed in 0.1M phosphate buffer, nonspecific binding sites were blocked by two hours of incubation in 5% normal donkey serum in 0.1M phosphate buffer with 0.5%
Triton X-100 (Block Buffer). Sections were then incubated overnight at room temperature with rabbit anti-STAT3 antibody (Santa Cruz Biotechnology) in Block
Buffer. The secondary antibody (biotinylated donkey anti-rabbit IgG) was detected using the Avidin-Biotin-Peroxidase kit (Vector Laboratory) with DAB as substrate. Sections
were then mounted on Superfrost plus slides (Fisher), dehydrated and coversliped with
Permount. Photos were taken at 300X magnification using a digital camera linked to the microscope.
Semi-quantitative RT-PCR. Hypothalami were isolated from ad libidum -fed mice in the eighth week of age between 1000 and 1200 hours and snap-frozen. Total hypothalamic RNA was isolated using Ultraspec reagent (Biotecx). For determination of relative RNA concentration, total hypothalamic RNA was subjected to automated fluorescent RT-PCR on an ABI 7700. Primers used were: ctgcttcagacctccatagatgtg
(forward POMC), cagcgagaggtcgagtttgc (reverse POMC), 6FAMcaacctgctggcttgcatccgg-TAMRA (probe POMC); tcagacctcttaatgaaggaaagca (forward
NPY), gagaacaagtttcatttcccatca (reverse NPY), 6FAM-ccagaacaaggcttgaagacccttccat-
TAMRA (probe NPY); cagaagctttggcggaggt (forward AgRP), aggactcgtgcagccttacac
(reverse AgRP), 6FAM-ctagatccacagaaccgcgagtctcgtt-TAMRA (probe AgRP). GAPDH control primers and probes were as supplied by ABI. Each predicted RT-PCR product spanned an intron/exon junction. Each RT-PCR reaction was determined to be in the linear range for quantitation by comparison to serial dilutions of input hypothalamic
RNA.
Reference List
1. Schwartz,M.W.
et al.
Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46 , 2119-2123 (1997).
2. Baskin,D.G., Breininger,J.F. & Schwartz,M.W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus.
Diabetes 48 , 828-833 (1999).
3. Hubschle,T.
et al.
Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J
Neurosci 21 , 2413-2424 (2001).