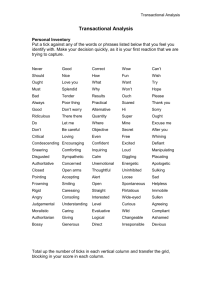
Purification affinity chromatography
advertisement

Affinity Chromatography Solutions needed: 1. 10-15mg of GAPDH from extract Stir your sample gently and withdraw small aliquot containing 10-15mg of protein. Centrifuge and decant the supernatant and remove any excess fluid with tissue paper. Dissolve the crystals in 0.5 -1.0ml column buffer. 2. 2-ml Affi –Gel Blue Ready to use form 3. 2g sephadex G-50 Add 50ml column buffer to 2g of dry sephadex gel. Let it swell for 3hr at room temperature or 1 hr at 90o C. occasionally stir the gel solution slowly and gently. 1g of dry gel swells to 9-11ml bed volume Degas the gel solution by water aspiration. 4. 2L 50mM sodium phosphate, pH 8.6 containing 1mM EDTA 0.1247g of monosodium phosphate monohydrate 13.1578g of disodium phosphate heptahydrate 0.292g of EDTA Final volume 1L 5. 20ml column buffer containing 2M NaCl 116.88g /1L is 2M 6. 20ml Column buffer containing 10mM NAD 63mg/1L is 10mM 7. 20ml Column buffer containing 1M NaCl 58.44g / 1000ml1M Desalting Crude extract: 1. Connect the column to UV monitor. 2. Pour the swollen matrix into a glass column and equilibrate the column with 5 bed volumes of column buffer. 3. Apply the protein solution prepared to the sephadex column 4. Elute with the column buffer. 5. Collect 1ml fractions and pool fractions that have 90% of the proteins Affinity Chromatography: 1. 2. 3. 4. 5. 6. 7. 8. Gel is ready to use. Mix gently by inverting the gel. Fill the small column with 2ml bed volume. Wash the column with 5 bed volumes of column buffer with 2M NaCl and 10bed volumes of column buffer. Apply the desalted GAPDH to the equilibrated gel and wash with several bed volumes until contaminating proteins do not elute. Elute GAPDH with column buffer with column buffer containing 10mM NAD (should elute in less than 5 bed volumes). Collect 1ml volumes. We can use UV monitor or Bradford method to estimate the total protein content.