LAB # 4
advertisement

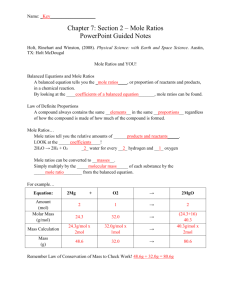
LAB # 5 - 2010 A QUALATATIVE STUDY OF MOLE RATIOS IN A CHEMICAL REACTION PRELAB: Review the definitions of any terms in italics in this lab. Read about mole to mole ratios in a reaction. OBJECTIVE: To prove the mole ratio that exists between the reactants is one to one as indicated by the reaction equation that describes the reaction studied in lab (the equation will be given to by your teacher): INTRODUCTION: This lab will introduce the concept of mole ratios between different substances in a reaction. The mole ratios are indicated by COEFFICIENTS in the balanced reaction. Some important facts about coefficient are: a) A coefficient precedes the formula it defines. b) They can be changed to balance the reaction. c) Coefficients are usually in the unit of the MOLE which is the universal unit of ratio between different substances in any chemical situation. d) If a substance has no coefficient in front of it, the coefficient is assumed to be one. The reactant compounds will be aqueous solutions of the same concentration. This means equal volumes of these solutions will have equal numbers of moles and therefore equal numbers of formula units. As you will measure the reactants in drops, the drop ratio is the mole ratio and therefore the coefficient ratio for the reaction. You will mix the reactants in various ratios; the exact ratio will give you the most solid product. SAFETY : Wear safety goggles and follow the instructions of your teacher. MATERIALS a) Aqueous ( 1 Molar) solutions of your reactants) . b) 2 micro pipettes. c) Microchemistry plates with small and large wells. d) Tooth picks for mixing. PROCEDURE 1) Obtain a pipette and fill it with the reactant _________; obtain another pipette and fill it with reactant ___________ . 2) Mix the two reagents by mixing drops according to the table. Be certain to use the line of wells near the edge of the plate such that you can see the results easily. NOTE – the wells are numbered on the plate and should correspond to the data table After all wells have been mixed, allow the plate to settle for about two minutes and observe relative heights of the solid product,_________. Indicate which well had the most precipitate. If your results are inconclusive, repeat the procedure. 4) Record your observations regarding the precipitate, and the solution above the solid precipitate, in each well. Table 1: Quantities of Reagents Used WELL # 1 _______(aq) 1 drop _______(aq) 9 drops 2 2 8 3 3 7 4 4 6 5 5 5 6 6 4 7 7 3 8 8 2 9 9 1 Post-Lab Analysis and Questions a) Did you prove the mole ratios in this reaction ? b) If 0.50 moles of ________ (s) was formed, how many moles of the _______ (aq) should have been used? c) If 2.0 moles of _________(aq) was used, how many moles of each of the other substances are involved. IMPORTANT – Make a model to simulate the reaction. Draw models that represent reactions in wells 2, 5 and 8 – be careful! Write the answers to these questions in your lab notebook. Draw the models in your notebook and have them checked by your instructor before the end of lab period.