Stoichiometry * Measuring chemical reactions
advertisement
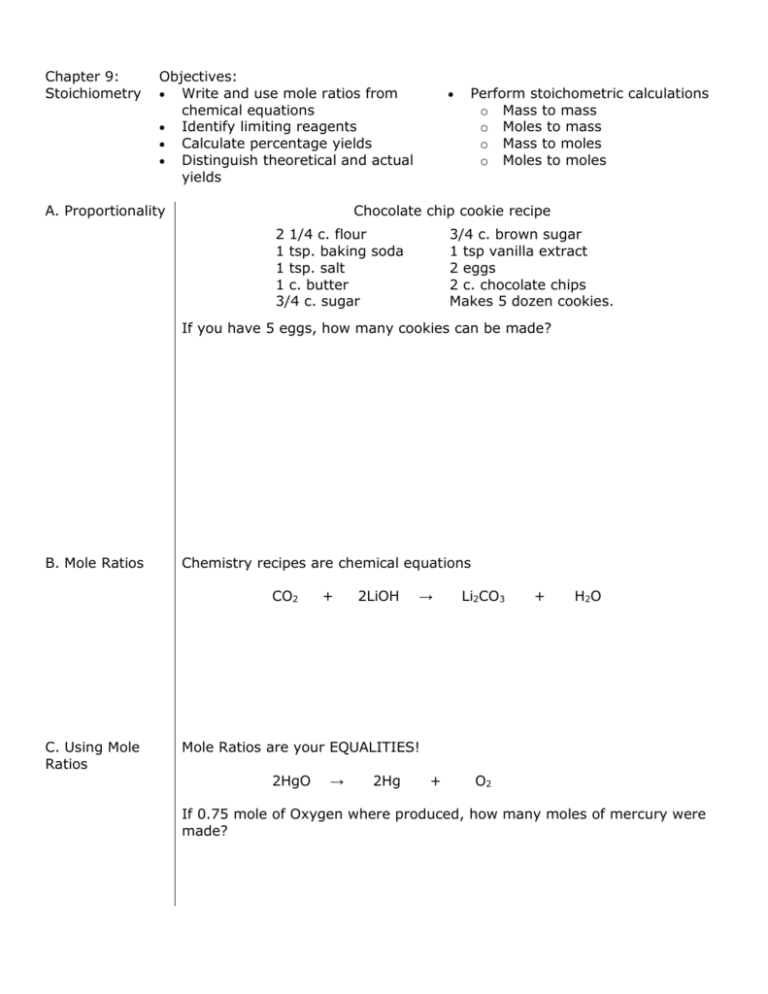
Chapter 9: Stoichiometry Objectives: Write and use mole ratios from chemical equations Identify limiting reagents Calculate percentage yields Distinguish theoretical and actual yields A. Proportionality Perform stoichometric calculations o Mass to mass o Moles to mass o Mass to moles o Moles to moles Chocolate chip cookie recipe 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. If you have 5 eggs, how many cookies can be made? B. Mole Ratios Chemistry recipes are chemical equations CO2 C. Using Mole Ratios + 2LiOH → Li2CO3 + H 2O Mole Ratios are your EQUALITIES! 2HgO → 2Hg + O2 If 0.75 mole of Oxygen where produced, how many moles of mercury were made? D. Stoichiometry problems 1. 2. 3. Mole to mole CO2 + 2LiOH → Li2CO3 + H2O How many moles of lithium hydroxide are required to react with 20 mol of CO2, the average amount exhaled by a person each day? Mole to mass 6CO2 + 6H2O → C6H12O6 + 6O2 How many grams of glucose is made if 3 moles of water react? Mass to moles 6CO2 + 6H2O → C6H12O6 + 6O2 How many moles of water are needed to react with 132 g of carbon dioxide? Mass to mass Sn + 2HF → SnF2 + H2 If you start with 30 g of HF, how many grams of SnF2 can you make? E. Limiting and Excess reactants Limiting Reactant Excess Reactant 1. 2. 3. 79 g of zinc react with 81 g of HCl. Identify the limiting and excess reactants. How many grams of ZnCl2 are made? Zn F. Percent Yield + 2 HCl → ZnCl2 + H2 What’s the percent yield if you collected 3.6 g of a compound and the stoichiometric amount is 4.5g? % yield actual yield x100 theoretical yield











