Jānis Jušinskis. Impact of vascular condition on hemodialysis and
advertisement

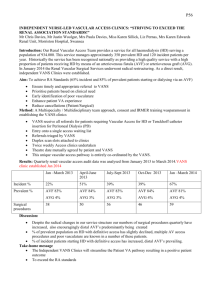
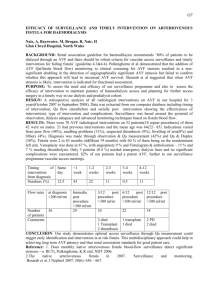
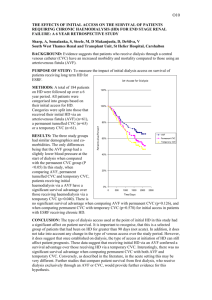
RIGA STRADINS UNIVERSITY Jānis Jušinskis IMPACT OF VASCULAR CONDITION ON HEMODIALYSIS AND RENAL TRANSPLANTATION OUTCOMES (SPECIALITY- TRANSPLANTOLOGY) Synopsis of thesis Scientific supervisors: Professor R. Rozentāls Ass. professor D. Krieviņš Riga - 2006. I. INTRODUCTION Renal Replacement Therapy (RRT) (hemodialysis, peritoneal dialysis and kidney transplantation) in patients with chronic renal failure (CRF) is one of the most challenging of hitech treatments. It requests for expensive hemodialysis devices, specific disposable blood circulation lines, peritoneal indwelling catheters, immunosuppressants, as well as extra attention provided by care givers, biomedics and patients themselves. Over 1 million patients all over the world are treated with RRT, and ca. 200 000 patients every year are primary ones. One of the ranges of critical issues is the problem of vascular access for permanent connections to "artificial kidney" - arteriovenous fistulae (AVF) and central venous catheters (CVC). AVF is the optimal vascular access solution both for patients and personnel, who are to repuncture the fistula repeatedly. However, establishing and maintenance of fistulae are associated with a range of critical complications, such as thrombosis of fistula in early postoperative period, as well as in several years (Miller et al, 1999). It is not rare that AVF happens to be underdeveloped after it is created, thus preventing its use for hemodialysis (HD) and leaving patients without vascular access, which is of life importance for them (Gibson et al, 200I). Attempts to replace AVF with synthetic AV grafts did not solve the problem, because just 58-74% thereof stays patent more than one year (Nicholson, Murphy, 2000). To establish vascular access in case HD is to be performed by vital indications in emergency while AVF is obstructed by thrombus or is not functioning properly, central venous cannulation is done with special CVC. Modern plastics allow for better catheter structure and longer indwelling time. As per information from Pisoni et al. (2002), CVC provides for permanent vascular access for HD in 20-30% of patients. However, CVC is associated with a range of serious adverse effects, mostly surgical ones. Allan M (2004) observed 7 times more frequent infections and bacteremia in patients with CVC than in those with AVF, while Little et al. (2001) found that 36.6% of cannula evacuations were associated with cannula thrombosis. The above drawbacks request for new evidence-based approach in solving the problem of vascular access. Routine clinical and anatomical examination of vascular status is not enough. Specific vascular examination methods should be used before creating the AVF. The most easy and non-invasive method is duplex dopplerography, which allows to evaluate vessels in details. The use of it before creating an AVF has been started in Latvia just few years ago. Although the duplex dopplerography is entered is National Kidney Foundation - Dialysis Outcome Quality Initiative Guidelines (NKF-DOQI 2006) examination list, it fails to provide comprehensive information. Thus, further investigations and developments are necessary. The same refers also to central vessel catheterization. Performing the procedure without due control and experience may result in increased incidence of complications. When examining vessels in uremic patients we found also high incidence of vascular complications in patients, who underwent the third of RRT methods - kidney transplantation (Cetingok et al., 2004; Seron et al., 2001). Actually, in patients with impaired kidney function pelvic and transplant vessels have same lesions, as peripheral vessels. This is one of possible causes of early (renal artery thrombosis) and late (renal artery stenosis, atherosclerosis) complications after kidney transplantation. Taking into consideration the above, we found it necessary to examine the vascular status of CRT patients undergoing RRT and develop the ways and methods of reducing complications. II. AIM AND OBJECTIVES OF THE STUDY Aim of the study: to improve quality of hemodialysis vascular surgery and renal transplantation. Study objectives: 1. To improve quality of AVF creation. 2. To improve methods of CVC implantation. 3. To design new double lumen CVC for HD. 4. To predict the outcome of kidney transplantation based on vascular status of recipient. III. STUDY NOVELTY We developed a general algorithm for duplex dopplerography of the hand vasculature, and developed the indicators, allowing to predict AVF functioning, as well as to define optimum placement of AVF. We designed new double lumen CVC for HD, allowing for unlimited cannula flushing with liquids, antibacterial and antithrombotic drugs between HD procedures, resulting in reduced bacterial colonization of lumens and infection, while preventing thrombosis of the catheter. Considering outcomes of AVF and kidney transplantation we found for the first time that risk factors of insufficient development and thrombosis of AVF are associated with kidney transplantation outcome, contributing to the development of chronic allograft nephropathy (CAN), increased incidence of graft loss and patient mortality in post transplantation period. IV. STUDY SCIENTIFIC AND PRACTICAL IMPORTANCE The developed algorithm for duplex dopplerography of the hand vasculature (arteries and veins) and the findings of the examination may contribute to significant decrease in the incidence of thrombosis and insufficiency of AVF, allowing for establishing proper native AV access in patients with CRF. Use of AVF considerably improves quality of HD, reduces incidence of hospitalization and increases survival rate in majority of the patients. CVC under ultrasound control is implemented into practice, which prevents intraoperative complications. The newly designed double lumen CVC for HD is meant to reduce post implantation complications during long-term use of central venous access. The new design allows for lumen flushing with liquids, antibacterial and antithrombotic drugs between HD procedures, resulting in reduced bacterial colonization of lumens and infection, while preventing thrombosis of the catheter. Decreased complication rate may considerably improve care quality, survival rate and costs, associated with CVC replacement and infection management in patients with CRF. Information on vascular impairments obtained during duplex dopplerography before establishing vascular access as well as outcomes of AVF creation may help select patients with increased risk of kidney transplant vessels sclerosis, which may result in transplant fibrosis and development of CAN. Thorough patient management after transplantation, as well as preventive therapy may decrease incidence of transplant rejection and increase life expectancy. Graft survival may be beneficial for "waiting list" by decreasing number of patients, who wait the grafting, thus allowing more CRF patients to undergo kidney transplantation. Unsatisfactory outcomes of AVF surgery provide us information on CAN risks and emphasize the importance of recipient non-immune factors for CAN development, which may be helpful in understanding the pathogenesis and prevention of the condition. V. APPROBATION OF STUDY RESULTS A report "Vascular access in HD". Latvian Association of Nephrologists session (2004); a report "Vascular lesions in patients with CRF and kidney transplantation", Latvian Association of Transplantologists session (2004); a report "AVF in HD patients", Latvian II Phlebology Congress (2005); a report "Vascular thrombosis after kidney transplantation", Latvian Association of Transplantologists session (2005); a report "Vascular access for chronic HD", Conference of P. Stradins university hospital (2006); a report "Color dopplerography resistance index as diagnostic criterion of acute and chronic graft rejection", Latvian Association of Transplantologists session (2006); poster report „Vascular access outcomes as a predictor of development of chronic allograft nephropathy", XXIII Congress of Scandinavian Transplantation Society (Göteborg, Sweden. 2006); a report „Use of ultrasound in establishing vascular access for HD", 15th Latvian Ultrasonography Conference (2006); poster report „Poor peripheral vascular condition as a negative predictive value in kidney transplantation", V Congress of Baltic Surgical Association (Pärnu, Estonia. 2006) VI. MATERIALS AND METHODS Study population The population of the study were CRT patients (N=477), who underwent surgery and treatment in Latvian Transplantation Center. 1. Improvement of quality of A VF surgery, CRF patients were prospectively involved in the study (N=190, mean age of 55 ± 14 years [13 - 82 years], 87 male patients, 103 female patients), who were scheduled for vascular duplex dopplerography of the ipsilateral hand before eventual native vascular AVF surgery between December 1st , 2001 and June 30th, 2005. The follow-up time was defined 12 months postoperatively; 2. Reduction of complications of central venous catheterization during establishment of HD vascular access 196 patients were prospectively involved in the study (N=196, mean age of 56 + 15 years [11 - 89 years], 89 male patients, 107 female patients), who were scheduled for central venous catheterization with HD CVC (N=255, thereof 179 traditional cannulation and 76 ultrasound controlled cannulations) between January 1st , 2003 and December 31st, 2004. The follow-up time after CVC was defined up to the first HD session; 3. Impact of vascular condition on outcomes of kidney transplantation. We used incidence of AVF thrombosis before grafting as an indicator of vascular lesions, influencing the outcomes of kidney allografting. Retrospective study involved all of the patients (N=91, mean age of 49 ±13 years [18-73 years], 46 male patients, 45 female patients), who had AVF created between January 1st, 1999 and December 31st, 2002, followed by primary kidney allotransplantation. The follow-up time was defined at least 36 months after transplantation. Patients who had HD for longer than 24 months before allografting were excluded from the study, as well as all cases of warm ischemia during preparation of donor, taking the organ or during transplantation, preexisting antibody (PRA) concentration before transplantation > 10%; history of blood transfusions before surgery, primary nonfunctional allograft. Patients (N=91) were divided into 2 groups according to AVF surgery outcomes: Group 1 (N=47) without AVF thrombosis, HD performed via AVF; Group 2 (N=44) AVF thrombosis requested additional surgical intervention (Table 1). TABLE 1. Patient groups: Group 1 with functional AVF; Group 2 with AVF thrombosis episodes. Group 1 Group 2 Patients: N 47 Mean age (years) Male/Female Mean HD duration (months) Mean follow-up time (months) 49.0 ± 14.1 (29-72) 28 / 19 44 50.0 ±12.3 (18-73) 18/26 (59.6% / 40.4%) (40.9%/59.1%) 9.9 ±5.0 (5-24) 10.5 ±5.9 (4-24) 40.6 ± 10.7(37-81) 53.9 ±12.2 (36-81) 38.5+11.5(16-63) 39.1 ±11.0(17-65) 33/ 14 30 / 14 Donors and surgery data: Mean age (years) Cause of brain death (trauma/other) Cold ischemia time (hours) (70.2%/29.8%) (68.2%/31.8%) 16.5 ±5.4(1 -25) 15.3 ±4.5 (4-24) Examining the patient before establishing AV vascular access Patient examination involved clinical and laboratory tests (history, examination of hand to undergo surgery, arterial pulse at the elbow and forearm, blood pressure, blood count and blood chemistry, blood clotting tests), X-ray and imaging (duplex dopplerography of the hand, shoulder and forearm, X-ray, venography, angiography, CT and MRI angiography). Duplex dopplerography of hand vessels before A VF surgery We used LOGIQ 400 CL device (General Electric) with linear 9 MHz transducer ("fine detail" or 'Vascular" program). In supine patients V. Subclavia was found under the clavicle, and then followed blood flow measurement by spectram (triple measurement, followed by mean value calculation) and analysis of breathing influence on the above flow. A. brachialis was examined in standing patient, starting from upper third of the shoulder to artery bifurcation. Thickness of vascular wall (sclerosis, stenosis, and calcinosis) was also checked and blood flow at elbow measured by spectrum. A. radialis (in standing patient) was examined along the whole length of it, measuring also the wall thickness, blood flow and internal diameter of the artery at different levels. The same procedure was done for A. ulnaris, to detect the dominating vessel. For subcutaneous vein (usually v. cephalica) the compression was checked, as well as its diameter at different levels (without tourniquet) and course of vein along the whole length of forearm. Examination findings were used to mark the most suitable AVF location. Creating AVF As a routine, we used distal third of forearm of non-dominant upper limb to create AVF by anatomizing a. radialis and subcutaneous vein (v. cephalica). Usually we used local infiltration anesthesia. Type of anastomosis was „end-to-end", but the diameter was 5 to 8 mm. Suturing was done with everted continuous 7-0 or 6-0 non-resorbable monofilament atraumatic vascular suture (Prolene). After anastomosing and hemostasis AVF was placed subcutaneously and observed for proper functioning. Unsatisfactory AVF function lead to revision of the vessel (kinking, branching) and eventual corrections. After having achieved due AVF function we inserted wound drainage and closed the wound. Indwelling double lumen CVC for HP CVC was performed under local anesthesia with the patient being in Trendeleburg position. Traditional CVC: The vein is at first positioned with thin injection needle, using anatomical landmarks. After that the vein is punctured with large-bore needle, followed by guide wire insertion. Ultrasound-guided CVC: We used LOGIQ 400 CL device (General Electric) with linear 9 MHz transducer, covered by sterile polyethylene bag. After visualizing the vein local anesthesia was done, then, watching the vein and the needle, venous puncture was performed. Guide wire was introduced via the needle under ultrasound control, followed by cannula insertion m. Seldinger. HD Routine HD procedure supposes 3 to 4 sessions per week, 3 to 5 hours long (depending on patient status and available blood flow). Kidney allografting Removing and preserving the donor kidney: Donor kidney is removed after verification of biological death or brain death of the donor. Donor organs are approached via median laparotomy access, with organ flushing technique in situ is used (before 2001 preserving was done by EuroCollins solution, since 2002 we use Custodiol (НТК). After removal the kidneys are examined macroscopically and placed in special containers with preserving solution. Donor kidney allocation was based on cross-matching and ABO-matching (blood groups) results; HLA matching was not considered. Immunological testing (Cross-matching, PRA level) were done in tissue typing laboratory of State Blood donor Center. Kidney allografting surgery was done in general anesthesia via pararectal incision in hypogastrium, pelvic vessels were approached retroperitoneally. Donor kidney vein was anastomosed to v. iliaca externa, but artery - to a. iliaca externa, stent was introduced into ureter, which was anastomosed to urinary bladder. Vascular anastomoses were done with everted continuous 5-0 or 6-0 non-resorbable monofilament atraumatic vascular suture (Prolene), urethral anastomoses - with 4-0 monofilament atraumatic suture (PDS). Posttransplant immunosuppression Induction immunosuppression: monoclonal antibodies (Simulect, Zenapax) or polyclonal antibodies (anti-T-lymphocyte globulin, ATG), methylprednisolone (Solu-Medrol) pulse therapy. Maintenance immunosuppression: steroids (prednisolone), calcineurine inhibitors (cyclosporine A) and antiproliferative drugs (mycophenolate mophetyl or azatioprine). Histology All of the histology tests were done at P. Stradina KUS Pathology Institute. Vascular histology was done by light microscopy; samples stained by hematoxyline-eosine and Van-Gieson, allograft biopsy histology assessed according to Banff (1997) classification Data processing and statistics Obtained data were stored in Microsoft Excel 2003 and Microsoft Access 2003 applications. Text and tables were processed in Microsoft Word 2003 application. Data analysis was done by SPSS 13.0 for Windows application (2004 SPSS Inc.). All of the values are presented as means ± standard deviation (SD). Statistical confidence was defined at p < 0.05. Interrelation between AVF thrombosis incidence, patient demographics and vascular duplex dopplerography findings was analyzed by multivariate test (general linear model). To find out correlation of thrombosis incidence and selected vessel parameters we used chi-square test, except patients, whose other vessels' parameters were associated with increased AVF thrombosis (v.subclavia blood flow < 400 ml/min, a.radialis diameter < 1.7 mm, subcutaneous draining vein diameter < 1.9 mm). Duration of AVF function was analyzed by Kaplan-Meier surveillance test. Correlation was analyzed by Pearson's Correlation test. Traditional CVC and ultrasound-controlled CVC were compared by chi-square test. Analysis of vascular status impact on kidney transplantation outcome involved all of the database entries for all vascular access options and/or kidney transplantations, performed at Latvian Transplantology Center from January 1st, 1999 till December 31st, 2005 (N=906). Information was obtained from Surgery Log records, "Lattransplant" coordination dept. and outpatient dept. database and archive. Posttransplant complications correlation to patient demographics and AVF outcomes was analyzed using multivariate test (general linear model). Allograft and patient survival rate was assessed using Kaplan-Meier survival test. VII. RESULTS 7.1.1 Improvement of quality of AVF surgery In 19 patients (10%) AVF had been canalled after duplex dopplerography, thereof 4 due to v.subclavia occlusion, 5 due to severe artery sclerosis (calcinosis) associated with stenosis/occlusion, and 10 due to subcutaneous vein thrombosis or occlusion (usually after multiple intravenous injections). Total of 192 AVF created, thereof 55 fistulae developed thrombosis, thereof 47 cases (24.5%) in early postop period (before use of AVF for HD), and 8 cases (4.2%) - after AVF use for HD. Overall AVF creation efficacy (usage for HD) was 75.5%. Mean number of functional AVFs (AVF surveillance) within a month was 81.3%, within 12 months - 72.9% (Fig. 1.). Fig. 1. Functional AVFs during 12 months (Kaplan-Meier survival curve). Statistics showed correlation of AVF thrombosis incidence with female gender (p < 0.005), v. subclavia blood flow (p < 0.01) and blood flow response to patient breathing (p < 0.001), a. radialis lumen diameter (p < 0.001) and AVF draining subcutaneous vein lumen diameter (p < 0.01). Correlation of thrombosis with age and diabetes mellitus was not found. V. subclavia mean blood flow was 1020 + 470 ml/min (from 150 to 2790 ml/min). Blood flow values were higher in functional AVF group compared to AVF group with history of thrombosis (1080 ± 500 vs. 880 ±510 ml/min, p< 0.01). V. subclavia decreased blood flow response to breathing was seen in 23 patients (12.0%) with negative correlation with v. subclavia blood flow (Pearson's Correlation = -0.46, p< 0,001). V.subclavia group with blood flow of less than 400 ml/min. AVF thrombosis was seen in 100% of patients (p < 0.001); this group also had no blood flow response to breathing. AVF thrombosis was statistically confident to occur in patients with v. subclavia blood flow of 400 to 450 ml/min (p = 0.010), especially when combined with insufficient blood flow response to breathing (p = 0.001). Blood flow of 450 to 500 ml/min did not show statistically confident correlation with incidence of thrombosis (Table 2). Table 2. Incidence of thrombosis at different v. subclavia blood flow values. Blood flow value (BF) BF ≤ 400 ml/min Fisher's exact test (p) Odds Ratio; 95% Confidence Interval < 0.001 400 ml/min < BF ≤ 450 ml/min = 0.010 7.53; 1.69 to 33.61 450 ml/min < BF ≤ 500 ml/min = 0.086 3.41; 0.86 to 13.61 A. brachial is mean lumen diameter was 3.6 + 1.0 mm (2.0 to 5.9 mm), blood flow 170 ± 90 ml/min. (30 to 640 ml/min.). We did not find statistically reliable difference in brachial artery flow in patients with normal AVF function and those with history of AVF thrombosis (0.17 + 0.09 vs. 0.15 ±0.08 L/min.,p = 0.136). A. radialis mean lumen diameter was 2.0 ± 0.4 mm (1.3 to 3.5 mm). Patients with normal AVF function had considerably larger artery diameter compared to those with history of AVF thrombosis (2.1 ±0.4 mm vs. 1.8 ±0.3 mm, p< 0.001). Statistically AVF thrombosis incidence was reliably higher when a. radialis was ≤ 1.5 mm (p < 0.001), relatively high at a diameter of 1.6 mm (p = 0.070, statistically unreliable). A. radialis diameter of 1.7 mm did not show higher AVF thrombosis risk (Table 3). The most frequent histological finding was fibrosis of intima and media with signs of atherosclerosis. Table 3. Incidence of thrombosis at different lumen diameters of a. radialis. A. radialis diameter Fisher's exact test (p) Odds Ratio; 95% Confidence Interval ≤ 1.5 mm < 0.001 10.05; 2.89 to 35.18 = 1.6 mm = 0.070 3.20; 0.92 to 11.17 = 1.7 mm = 0.135 2.36; 0.72 to 7.72 Mean lumen diameter of subcutaneous draining vein was 2.4 ± 0.6 mm (1.4 to 5.6 mm). Impaired venous flow (by compression test) in one of its segments was found in 14 patients (8.2%), which were considered when determining AVF level (proximally to occluded segment). Patients with normal AVF function had bigger subcutaneous draining vein diameter than those with history of AVF thrombosis (2.5 ± 0.6 mm vs. 2.3 ± 0.5 mm,p < 0.01). The AVF subcutaneous draining vein diameters of ≤1.6 mm and 1.7 mm were statistically reliably associated with higher risk of AVF (resp.,p = 0.002 and p = 0.026). Group of patients with subcutaneous draining vein diameter of 1.8 mm had no increased risk of AVF thrombosis (Table 4). The most frequent histological finding was associated with intima proliferation and fibrosis. Table 4. Incidence of AVF thrombosis at different diameters of subcutaneous draining vein. Diameter of subcutaneous draining vein Fisher's exact test (p) Odds Ratio; 95% Confidence Interval ≤1.6 mm = 0.002 9.85; 2.31 to 41.92 = 1.7 mm = 0.026 4.67; 1.26 to 17.25 = 1.8 mm = 0.370 1.59; 0.39 to 6.37 In 69.1 % of cases AVF thrombosis was seen in patients, whose vascular parameters (by duplex dopplerography) were associated with higher risk of thrombosis, while total of 77.0% of high risk cases of AVF thrombosis were detected by duplex dopplerography (including patients with cancelled AVF after duplex dopplerography). Analysis of AVF surgery in our Center showed that after duplex dopplerography had been introduced in 2002, the number of AVF reconstructions considerably reduced (Fig 2). Fig. 2. Total number of AVF and AVF reconstructions due to thrombosis in Latvian Transplantology Center in 2001.-2005. We can conclude from the above that use of duplex dopplerography before AVF surgery may provide us meaningful information on future AVF function and help us avoid failed interventions. Definite vascular parameters, associated with higher risk of AVF thrombosis, may help us develop guidelines for vascular access in HD patients. 7.2. Reduction of complications of central venous catheterization during establishment of HP vascular access. 7.2.1. Ultrasound controlled CVC insertion 179 of 255 CVCs were performed in traditional way (thereof 74 indwelling, 105 temporary CVC), while 76 cases were ultrasound-controlled (thereof 34 indwelling, 42 temporary CVC). Ultrasound-controlled CVCs were performed in patients with history of multiple or failed central venous catheterizations (N = 59) or just after failed traditional CVC (N = 17). In 41 (22,9%) of 179 CVC traditional procedures the insertion of cannula was successful only after the puncture location was changed (switching to other vein), thereof in 6 cases (7.6%) v.subclavia was used via subclavicular route. 20 of CVCs (11.2%) failed; however, ultrasoundcontrolled CVC was successful in 100% cases without switching to other vessel (p = 0.001). First attempt CVC was successful in 107 cases (59.8%) using traditional technique and in 73 cases (96.1%) using ultrasound control (p < 0.001). Such complications as puncture of a. carotis, local haemotoma and bleeding from puncture canal were more frequent with traditional CVC technique (Table 5). Table 5. Efficacy of CVC implantation and number of complications. Traditional CVC N 179 US-controlled CVC Chi-square test, pvalue. 76 Efficacy 159 (88.8%) 100% p = 0.001 First attempt CVC 107 (59.8%) 73 (96.1%) p< 0.001 Switching to other vessel after failed CVC 41 (22.9%) 0 (0%) p< 0.001 Bleeding from CVC tunnel 11 (6.1%) 1 (1.3%) p = 0.082 Arterial puncture 24 (13.4%) 0 (0%) p< 0.001 Haemotoma 18(10.1%) 0 (0%) p = 0.001 During the study we did not face such complications as pneumothorax, haemothorax or/and haemomediastinum, damage of plexus brachialis or n. recurrens laryngis. In 35 cases CVC was introduced to v.subclavia and v. jugularis externa or v. jugularis interna confluence point, which provided for better venous blood flow along the cannula and prevented from cannula kinking in subcutaneous canal, as well as extended a range of possible locations for other central venous punctures, protecting patients from v. subclavia cannulation via subclavicular route.. Summarizing the results, we can see that intraoperative ultrasound control during venous puncture and guide wire introduction considerably improves efficacy of the puncture and reduces the risk of cannulation-induced complications. We would recommend this cannulation technique for all of the patients scheduled for HD cannula insertion. 7.2.2. Reduction of infectious and thrombotic complications of CVC for HD Long-term HD via CVC is associated with higher risks of bacterial colonization of inner surface (lumen) of catheter and bacteremia, as well as catheter thrombosis. These complications always lead to catheter removal, which limits vascular access for HD. The risks of bacterial colonization of lumen of catheter, as well as catheter thrombosis during long-term use may be reduced by flushing the catheter with antiseptic and thrombolitic drugs and bigger volumes of IV solutions. Current design of CVC does not allow for such options, as flushing solution gets into patient's blood (Fig 3). Fig 3. Design of short-term central venous double lumen catheter for HD (longitudinal lumen axial section): 1 - external part; 2 - intravenous part; 3 - arterial line; 4 - venous line; 5 -external openings of the lumens; red and blue lines show blood flow in the cannula during HD. In order to reduce bacterial colonization and thrombosis of the catheter the design of it should allow for both performing the HD (to collect blood from vein, pump it through HD device circuit and return to the same vain centrally to blood collection point) and easy flushing the catheter between HD sessions (the solution is flushed via external opening of one of the lumens, rinses both lumens along the whole length of those and returns via external opening of the other lumen without getting to patient's blood). We would like to offer the new design of the CVC for HD, able to provide the above options. The design is possible due to the following CVC upgrades: 1. The arterial lumen of CVC shall be extended (it should be 5 to 7 mm longer than venous one) with distal opening left open; 2. The external wall of arterial lumen (opposite the venous lumen) shall have an opening for collecting blood form the vein; 3. Distal opening of venous lumen shall be closed; 4. Arterial and venous lumens shall have a communication at the distal part of intravenous portion of CVC; 5. An additional catheter shall be inserted in the arterial lumen with its external wall fitting close to internal wall of arterial lumen; the catheter can be moved along the longitudinal axis of arterial lumen, (the "third" mobile lumen); 6. The distal tip of the "third" lumen shall be closed, but the walls of it shall have two openings: the first is to match the opening in arterial lumen (to collect blood), but the second opening is to match the communication between arterial and venous lumens. The "third" lumen openings are designed in a way to allow its first opening match the arterial lumen opening, while its closed distal tip stays proximal to the communication of arterial and venous lumens. The second opening of the "third" lumen can be matched with the point of communication of arterial and venous lumens when closed distal tip of the "third" lumen blocks the very distal tip of arterial lumen. During HD session the 'third" lumen is placed with its closed distal tip proximal to the communication of arterial and venous lumens, thus dividing the arterial line into two parts. The HD device is connected to the external openings of the "third" lumen and venous lumen. Blood is being collected from the vein via matched arterial lumen and "third" lumen openings and gets to HD device via the "third" lumen. Blood is being returned via venous lumen first, then via communication point of arterial and venous lumens openings it gets to open distal tip of arterial lumen (Fig. 4,„A"). When flushing the cannula between HD sessions the "third" lumen is pushed forward to block with its closed distal tip the distal opening of arterial lumen. During flushing the solution is being injected to venous line, and then it goes to the "third" lumen via the communication between arterial and venous lumens and matching opening of the "third" lumen, then follows the "third" lumen and vents from the external opening of arterial lumen. (Fig. 4, „B"). Fig. 4. Updated (temporary) CVC design (longitudinal section, A - HD positioning, В -standby position): 1 - external part; 2 - intravenous part; 3 - arterial line; 4 - distal opening of arterial line (in „A" position it is communicated to venous line for blood return, in „B" position it is blocked by the closed tip of "third" lumen); 5 - opening in the external wall of arterial line (to collect venous blood, in „A" position the opening matches the first opening of the "third" lumen, in „B" position it is closed by the wall of "third" lumen); 6 - venous line; 7 - closed distal tip of venous line; 8 communication between arterial and venous lines (connecting both), in „A" position to return blood, in „B" position to flush the catheter); 9 - the "third" mobile lumen; 10 -closed "third" lumen distal tip; 11 - opening in the external wall of the "third" lumen („A" - to collect blood, „B" - closed by the wall of arterial line); 12 - the second opening of the "third" lumen (in „A" position closed by the wall of arterial line, in „B" position opened for catheter flushing). This design allows for both performing HD and flushing the catheter between HD sessions with necessary amount of flushing solution, also with antibacterial and antithrombotic drugs added, because the solution does not get into circulation and cannot induce hypervolemia and drag toxicity. HD catheter flushing, as well as catheter separation from patient's blood, may reduce infections and thrombosis of CVC lumen, thus extending the lifetime of CVC and reducing the number of its reinsertions. 7.3. Impact of vascular condition on outcomes of kidney allografting In order to clarify the impact of vascular status on outcome of kidney transplantation we used the incidence of AVF thromboses in pretransplant period as an indicator of vascular lesion. Posttransplant complications Comparison of two patient groups showed us no difference in terms of age, PRA level, HLAnon-matching, diagnosis, age of donor, cause of death of the donor (trauma/other), and cold ischemia time. The second group had some more female patients (p=0.095, not significant). Post transplantation complications were considerably higher (except acute rejection) in AVF thrombosis group (Table 6). Acute rejection episodes were seen in 11 patients in Group 1 and in 15 patients in Group 2 (23.4% vs. 34.1%, statistically not significant). Delayed graft function (necessity to perform at least one HD after transplantation) was seen in 1 patient in Group 1 and in 6 patients in Group 2 (2.1% vs. 13.6%,p = 0.030). Chronic allograft nephropathy (CAN) (except cases of allograft loss in early postop period) was found in 5 patients in Group 1 and in 11 patients in Group 2 (11.1% vs. 28.2%, p = 0.043). CAN incidence was higher in patients with acute rejection episodes (p = 0.003), diabetes mellitus (p = 0.045), delayed allograft function (p = 0.096, statistically not significant). During the study we observed total of 20 lost allografts (except patients who died with functional allograft), thereof 4 patient from Group 1 and 16 patient from Group 2 (8.5% vs. 36.4%, p = 0.002). The most common reason of graft loss was CAN (p < 0.001), although we noticed also association with diabetes mellitus (p = 0.039). Histology of removed allograft showed severe nephrosclerosis and arteriosclerosis. We faced 2 patients death in Group 1 and 8 patient deaths in Group 2 (4.3% vs. 18.2%, p = 0.046). Causes of deaths were associated with diabetes mellitus (p = 0.002), acute graft rejection postoperatively (p = 0.029), CAN (p = 0.001) and loss of allograft (p = 0.007). Table 6. Postoperative complications. (Group 1 - patients with functional AVF, Group 2 patients with history of AVF thrombosis; - CAN calculations censored for graft loss in early postoperative period). Group 1 (N = 47) Group 2 (N = 44) p- value Acute rejection. N 11(23.4%) 15(34.1%) NS Delayed function, N 1 (2.1%) 6(13.6%) 0.030 CAN , N 5(11.1%) 11 (28.2%) 0.043 Graft loss, N 4 (8.5%) 16(36.4%) 0.002 Patient death, N 2 (4.3%) 8(18.2%) 0.046 Kidney allograft and patient survival Kidney allograft 3 year survival (Kaplan-Meier survival estimate) was statistically significantly higher in functional AVF Group (Log Rank = 5,939, p = 0,015) (Fig. 5). One year allograft survival was 95.7% in functional AVF Group and 82.9% in AVF thrombosis Group, three year survival was 91.3% and 70.7%, respectively. Also patient survival was considerably higher in functional AVF Group (97.9% vs. 86.4% in AVF thrombosis Group) (Log Rank = 4.268, p = 0.039) (Fig. 6). Fig. 5. Allograft 3 year survival (Group 1 - functional AVF, Group 2 - history of AVF thrombosis. Fig. 6. Patient 3 year survival (Group 1 - functional AVF, Group 2 - history of AVF thrombosis). Study results show that patients with pre-transplantation AVF thromboses had much higher risk of postoperative complications, including loss of allograft and patient death. It can be explained by CRF-induced vascular remodulation and sclerosis, which may increase the risk of both AVF thrombosis and kidney allograft arteriosclerosis and nephrosclerosis. VIII. CONCLUSIONS 1. Duplex dopplerography of upper limb vessels is mandatory for patients with CRF, scheduled for AVF creation. 2. In 77% of AVF thrombosis cases patients had duplex dopplerography findings, associated with increased risk of vascular thrombosis. 3. It is important to evaluate v.subclavia blood flow and its response to patient breathing: diameter of a.radialis lumen; status of a.brachialis and a.radialis vascular walls; diameter, patency and course of subcutaneous draining vein when creating the AVF. 4. Use of ultrasound control for central vein cannulation allows reducing complications of the intervention. 5. The new design of the central venous catheter for HD may provide adequate vascular access for HD and reduce the risk of CVC lumen infections and thrombosis. 6. Data on history of AVF thrombosis in recipient is predictive for both patient and allograft survival. 7. Vascular lesions in patients with chronic renal failure (arterial and venous sclerosis) are risk factors for the development of CAN. IX. RECOMMENDATIONS FOR PRACTICE In order to avoid central vein catheterization and preserve patients' vessels for future vascular access, AVF should be created accurately and timely. Duplex dopplerography of patient vessels may provide useful information on vascular status and possibility to create AVF when scheduling a patient for AVF creation. Observing the following criteria may help considerably reduce the risk of postoperative thrombosis and insufficient development of AVF: • Blood flow breathing; in v.subclavia higher than 450 ml/min with good response to patient • Blood flow in a. brachialis and diameter of its lumen do not influence the incidence of AVF thrombosis, still it is important to evaluate whether vascular wall is able to dilate and provide increased blood flow; • Diameter of a.radialis lumen more than 1,7 mm, still it is important to evaluate whether vascular wall is able to dilate and provide increased blood flow; • Diameter of subcutaneous draining vein lumen more than 1,8 mm; vein should be patent (compression test), the course of vein should be undisturbed up to the elbow; • Preoperative upper limb vascular „mapping" can help find optimal surgical approach and locate AVF; It is recommended to perform CVC in patients with CRF only under intraoperative ultrasound control, because it reduces both the number of complications and vessel trauma. Application of the new design of catheter to production and practice could reduce the risk of infection and associated bacteremia and sepsis, as well as the risk of AVF lumen thrombosis, thus extending the lifetime of catheter and reducing morbidity and mortality of patients. Information on vascular status and vascular access complications in potential recipient may help to predict the higher risk of development of CAN postoperatively and to ordinate preventive therapy in order to reduce the incidence of allograft loss and patient mortality. X. Publications of study results (original papers) 1. Bicāns J, Ševeļevs V, Truškovs S, Jušinskis J. Asinsvadu pieeja regulārās hemodialīzes nodrošināšanai. Grāmatā Hemodialīze Latvijā - 2000 (Red. Rozentāls R); Latvijas Nieru fonds, Rīga, 2000. - lpp. 42-56. 2. Jušinskis J, Truškovs S, Suhorukovs V. Dupleks dopplerogrāfijas izmantošana pirms hemodialīzes arterio-venozās fistulas izveidošanas. RSU Zinātniskie Raksti (2004): 255-258. 3. Jušinskis J, Suhorukovs V, Truškovs S. Hemodialīzes centrālo vēnu katetru implantācija ultrasonogrāfijas kontrolē. Latvijas Ķirurģijas Žurnālā (2004) 4: 82-85. 4. Tnishkov S, Jushinskis J, Suhorakovs V. Enhanced criteria donors: Impact of cold ischemia time in "old-to-old" kidney transplantation. Latvijas Ķirurģijas Žurnāls (2005) 5: 14-16. 5. Jušinskis J, Rozentāls R, Bicāns J. Patent "Centrālais venozais katetrs" Nr. P-05-145, accept 31.03.2006. 6. Jushinskis J, Bicans J, Suhorakov V, Trushkov S, Rozental R. Vascular access outcomes as a predictor of development of chronic allograft nephropathy. Transplantation Proceedings (2006) 38 (8): 2657-2658.