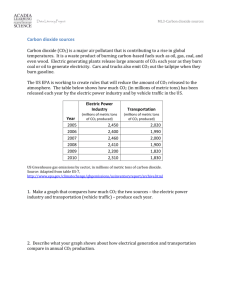
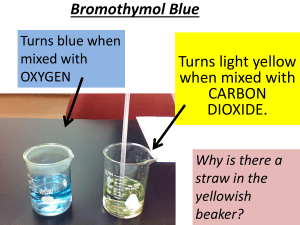
200 Jahre CO2-Gasanalyse der Luft mit chemischen Methoden
advertisement

History of CO2 Gas Analysis of Air by Chemical Methods Dipl. Biol. Ernst-Georg Beck StD, 31 Rue du Giessen, F-68600 Biesheim, egbeck@biokurs.de, 3/2007 Content Part 1 1. Summary of actual knowledge on CO2 gas analysis in air (2006) p. 3 Part 2 2. Methods of CO2 gas analysis in air 2.1 2.2 2.3 2.4 p. 15 Relative IR spectroscopic determination of the background level of carbon dioxide since 1958 Direct chemical determination of local effective CO2 concentration prior to 1958 Methods of historical CO2 gas analysis in air by chemical means Sources of errors and summary p. 15 p. 16 p. 23 p. 33 Part 3 3. Results of chemical CO2 gas analysis in air 1800 –1961 p. 34 3.1 Data of historical CO2 gas analysis in air by chemical means 3.2 Correlation of CO2-concentration with surface temperature 3.3 increase of CO2 between 1880 and 1927 Müntz p. 52, Heine p. 54, Spring p.54, Uffelmann p. 56, Petermann p. 57, Heimann/v.Frey p. 57, Letts&Blake p. 60, Brown&Escombe p. 61, Benedict p. 62, Lundegardh p. 63, summary, p. 66, Montsouris p. 67 p. 34 p. 49 p. 52 3.4 Verification of the CO2 maximum 1942 p. 68 Kreutz p. 68, Duerst p. 70, Buch p. 72, Lockhart p. 72, Misra p. 73, Hock/Scholander p. 74, Scandinavian Network p. 75, Steinhauser p. 76, summary p. 77 Part 4 3.5 Verification of further CO2-maximas 1857 and 1876 p. 78 v. Gilm p. 79, Schulze p. 79, Haesselbarth p. 81, Farsky p. 83, Reiset p. 84, summary p. 87 3.6 Evaluation of the CO2 characteristics before 1857 p. 88 de Saussure p. 89 3.7 Monthly cycling of carbon dioxide content according to lunar phases p. 91 4. Discussion p. 93 5. Conclusions P. 100 6. References P. 102 Continuing with PART 4 2 , 3.5 Verification of further CO2-maximas 1857 and 1876 78 Part 4 3.5 Verification of further CO 2-maximas 1857 and 1876 Twentynine yearly averages, chemically determined are used to contruct CO 2 concentration between 1857 and 1880, two are interpolated and nine resulted on measuring series lasting several month and years: v. Gilm, Schulze (2x), Smith, Reiset (2x), Farsky, Haesselbarth und Montsouris. Predomination measuring procedure was the Pettenkofer method partly modified. Duration of analysis of one sample was up to 24 hours conducted by Reiset. Measurements from different authors differ in regularity of sampling (irregular days a month or time a day) which resulted in distorted diurnal and seasonal variation and comparison between scientists is difficult. Sampling of Schulze, Farsky, and Haesselbarth was mostly daily at the same time and conducted by a tube through the window of analysing laboratory of the agrocultural station so possible errors of contamination are excluded. Smith used the Petterkofer method ( oxalic acid for titration) and sampled a comprehensive amount of data throughout England and Scotland in cities and rural areas. The data sampled in rural Scotland are of special interest [206]. Fig. 76 shows uncorrected CO2 concentration since 1857 up to 1880 determined chemically. Since 1857 used methods were calibrated against each other resulting in maximally average accuracy <= 3% (since v. Gilm 1857). Reconstructed curve fell down since 1857 from approx. 400 ppm to a low of approx. 300ppm around 1870 and rises again up to 1876forming a new little maximum. Despite beeing in error range of 3% this little maximum correlates with the IPCC temperature maximum and the 1857 CO 2 maximum fits to the GHCN temperature peak in 1857 (see fig. 33). Fig. 76 Envelope of reconstructed CO2 concentration from 29 yearly averages chemically determined in Europe 1857 – 1880 uncorrected showing maximum around 1857 und 1876. CO2 maxima around 1857 and 1876 are essentially based on the analyses conducted by v. Gilm (Innsbruck, 1856/57, corrected average: 383 ppm), Schulze (Rostock, 1863/64, corrected average: 351 respectively 361 ppm), Smith ( Perth, Scotland 1866, 336 ppm), Henneberg (Weende, 1872, 320 ppm), Farsky (Tabor, 1874, 346 ppm) and Haesselbarth, Dahme 1874, 330/340 ppm). With the exception of Smith there are comparing tests researchable of all other authors. Data from Tissandier 1875 (one day in march), Claesson 1876 (31 values in 1 month) and Montsouris 1877 (1 year with remarkable low values) are not included in Fig. 76. Measuring series at Montsouris (Paris) are not reviewed here referring to Stanhill [112]. 3.5 Verification of further CO2-maximas 1857 and 1876 79 Fig. 77 CO2 gas analysis of air in the garden of his institute of university of Innsbruck (Austria) conducted by H. v. Gilm from november 1856 to march 1857( 19 samples). Full moon: 12-11-1856, 11-12-1856, 101-1857, 9-2-1857 [191]. The chemist Hugo v. Gilm worked under the famous austrian chemist Hlasiwetz and used one of the accuratest methods at that time [104]). V. Gilm was the first researchable scientist, who tested his analysing procedure against air with known CO2 content achieving an accuracy of +-2,8%. Thereby he checked amount of CO2 absorption in Baryta Water. Pettenkofer himself, who experimentated in parallel compared accuracy of his titration method to v.Gilm´s procedure ( [58] and [138], p. 178). A test in winter 1858 (?) in Munich results in 452 ppm. Comparing measurements to the method of v. Gilm result in a deviation of 3,5%. The measuring series (6 month, 19 samples) in winter 1856/57 in Innsbruck displayed clearly the seasonal winter maximum in december 1856 with approx. 435 ppm. In march of the following year only 390 ppm was measured. Combining a guessed Innsbruck summer average 1857 of 350 ppm with a winter average of 416 ppm we can calculate a yearly average of 383 ppm (see table 4 ). Despite the few samples analysed by v. Gilm quality of sampling and analysing procedure is comfirmed by displaying monthly cycling with 3 corresponding CO2 maxima at full moon. Daily sampling of air by the chemist Prof. Franz Schulze in the Agricultural Experimental Station in periphery of Rostock, Baltic Sea, Germany 1863/64 [139] show accurate seasonal variation [140]. 3.5 Verification of further CO2-maximas 1857 and 1876 80 Fig. 78 CO2 gas analysis of air in Rostock, Baltic sea by Franz Schulze 1863-64. Full moon [191]: 11-251863, 1-23-1864, 4-22-1864, 5-21-1864, 8-17-1864, 9-15-1864, 10-15-1864, 11-13-1864, 12-13-1864. Fig. 79 Location of Agricultural Station Rostock (Baltic Sea) outside the city (above photo of the city of Rostock 2006 [139]). Above right corner: Franz Schulze, Prof. in chemistry and pharmacology 1850 –73 University of Rostock [139] The late autumn in 1863 at Rostock was warmer compared to 1864 (140). Regularly sampling in 13 month show a rise in CO2 concentration which corresponds to the contour of temperature (IPCC and other sources). Furtheron again monthly cycling is evident and the correlation to lunar phases is striking. 3.5 Verification of further CO2-maximas 1857 and 1876 81 Average of all samples is 359,7 ppm. Schulze was in doubt of the precision of his measurements because of high level of values and hesitated in publishing his findings. At the same time R.A. Smith measured in several rural areas of Scotland similar values and a summer (august, september) average level in 1865 of 336 ppm using Pettenkofer method [206]. Schulze examined was in doubt of his procedures leading to a new measuring series in 1868 to 1871. ([140], vol. 14, 1871, p. 366) These analyses using a modified Pettenkofer process and conducted in university of Rostock by sampling air through a tube in the window of laboratory 6-7 m above ground ( see position of university within historical city (exact location: Bluecherplatz [140]) in fig. 79 display also seasonal variation however with a level of approx. 50 ppm lower. Accuracy since 1870 was especially high. Three years average was 291,3 ppm. Again rising contour fits to rising temperature and monthly variation to lunar phase. Fig. 80 CO2 measurement of air in Rostock (Baltic Sea) by Franz Schulze 1868-71 [140] compared to IPCC temperature showing monthly cycling. Full moon : 10-1-68, 4-26-69, 6-24, 8-22, 11-12, 1-17-70, 317, 7-12, 10-9, 12-8, 5-7-71, 5-5. Schulze discussed in detail influence of weather and wind eg. from the baltic sea containing less CO 2. Between 1874 and 76 P. Haesselbarth and J. Fittbogen [141] conducted daily CO2 measurements in the Agricultural Experimental Station at Dahme (Prussia, near Berlin). Franz Farsky had visited Haesselbarth and analysed air Tabor [142] (Bohemia) at the same time. Both used Pettenkofer method by Schulze Rostock. 3.5 Verification of further CO2-maximas 1857 and 1876 82 Fig. 81 Location and 1940 view of Agricultural Experimental Station at Dahme (Prussia near Berlin, [143]) founded in 1857 by Hermann Hellriegel. Hellriegel, director of Station at Dahme had discovered root-fixation of N2 by bacteria in 1886. Procedures and equipment wer nearly identical used by Haesselbarth and Farsky sampling air by a tube through laboratory window. Haesselbarth did air sampling every morning between 8.30 am and 11.30 am in 2,85 m height from courtyard of Agricultural Station in periphery of the small rural town of Dahme (Prussia), which was in former times a center of agricultural research. Abb. 82 Diurnal CO2 variation measured on 24th /25th of july 1876 at Dame (Prussia) by Haesselbarth Additionally careful protocolling of several meteorological data by Haesselbarth can be researched. Since 1875 Fig. 83 display a typical seasonal contour. Because of starting his measurements in july a precise judgement of the year 1874 is difficult. Accuracy of work can be seen in several analyses of diurnal variation of CO2 (fig. 82) and minimal amplitude varaitions (fig. 83). Care, reliable Pettenkofer method and regularly measurements of 347 samples in rural location do no doubt quality of data despite distorted seasonal variation showing an average of 334 ppm. 3.5 Verification of further CO2-maximas 1857 and 1876 83 Fig. 83 CO2 analysis of air near Dahme (Prussia) by Haesselbarth and Fittbogen 1874/75. Full moon: 1015-1874, 12-23, 8-17-1875, 10-14. F. Farsky conducted 295 measurements during same years as Haesselbarth 1874/75 in periphery of little bohemian town Tabor ( today Czechia) [142] at the Agricultural Station. He started his analysis of nearly daily samples according the schedule of Schulze and Fittbogen and several tests in october 1874. Samples are collected in rural location in spring and summer in the morning and autumn and winter in the afternoon by a tube through the window of a building facing a rural street in free surround at aheight of 4,5 m in eastern direction. Fig. 84 CO2 analysis of air near Tabor ( today Czechia) by F. Farsky 1874/75 [142]. Full moon: 10-251874, 11-24,12-23, 2-20-1875, 3-21, 4-20, 5-20, 7-18 3.5 Verification of further CO2-maximas 1857 and 1876 84 Fig. 85 Old agricultural school outside Tabor (Cz) [169, 142], location of measurements by Franz Farsky 1874/75 Out of almost daily measurements a 10-days-average (decade) was calculated and plotted. Fig. 84 displays seasonal variation and full moon maxima as the accuracy of the Pettenkofer method with a yearly average of 346 ppm. 1872/73/75 and 1879 French chemist Jules Reiset conducted CO2 measurements near Dieppe (Normandy, North Sea, France) and Paris. 1872/73 analyses of 92 samples are conducted in his field station 8 km southern to Dieppe, at a height of 96 m above ocean level, resulting in an average of 290 ppm. 27 measurements in a deciduous forest resulted in an average of 292 ppm; in the field with blooming red clover he measured in june the average of 289,8 ppm and in 30 cm above ground in a field of barley in july 281,9 ppm. In Paris Reiset measured in 1873, 1875 and 1879 in the street of Vigny in may: 302,7 ppm. ( all values averages) Please notice unusual low values in the fields with high soil respiration. In [66] 1879 he described the very high carbon dioxide levels measured the past and documented in literature as 400 – 600 ppm and a strong fluctuating concentration in air.) He constructed sophisticated portable analysers using the Pettenkofer procedure with a huge volume of aspiration of 600 l (Reiset´s tower) for absorbing air in Barium Hydroxide titrating resulting carbonate by sulfuric acid. In contrast to german and other scientists air was dried by flowing through a U-tube with sulfuric acid ( see fig. 86). Reiset did not realize that this results in too low values because of CO 2 absorption in H2SO4 ([181] p. 83; Bunsen absorption coefficient H2SO4 at 25°C = 0,96; H2O at 25°C=0,759; [198]) These systematic errors were known since 1848, Hlasiwetz [103] 1856 and Spring [181] 1885 determined these absorption losses to 7-10% or about 20 ppm. I cite here the refering sentence out of reference [144], p. 165: Fig. 86 Scan of reference [144] showing proof of drying air by sulfuric acid in Reisets equipment 3.5 Verification of further CO2-maximas 1857 and 1876 85 Fig. 87 Location of field station in Dieppe (France) of J. Reiset 1872-80 and first part of analysing equipment (144) In Reisets mobile analyser samples are drawn at aheight of 4 above ground, whole anlysis procedure lasts beetween 7 and 24 hours, average 9 hours. Abb. 88 Mobile CO2 gas analyser of J. Reiset used near Dieppe (F) 1872-80 including tower like aspirator (2 m) with a volume 600 L (144) In (144, 1882) he precisly described his apparture and careful procedure. Unfortunatedly he did not measur at regularly times sometimes during daytime then at night and not daily. The result is seen in fig.89 showing a distorted image of seasonal variation and no diurnal fluctuation is researchable. Reiset knows diurnal and seasonal variation from de Saussure and Boussingault nevertheless he critisized de Saussure for his high level values as Marie-Davy of Montsouris observatory Paris for his strongly fluctuating values within 4 years 1877 to 1880 and Schulze for his contradicting results measured in 1868-71 accusing him using a erroneous method. This though Schulze´s average in 18681871 of 291 ppm was almost identical to Reiset´s 294 ppm für 1872/73 war. He claimed Schulze´s values in 1863/64 as wrong furtheron he tried to contradict Schulze´s postulate that wind direction and absorption capacity of the ocean influence carbon dioxide concentration if air. In total Reiset´s comments show the concurrence of French and German scientists at war times in 1870/71 to get scientific honours. Because of procedural and systematic errors only some data of Reiset are presented here. 86 3.5 Verification of further CO2-maximas 1857 and 1876 Fig. 89 Analysis of 92 air samples near Dieppe (Normandy, France) by J. Reiset in 1872/72. Full moon: 7-20-72, 2-12-73, 3-14, 4-12, 5-12, 6-10, 7-10. Reisets data sampled 8 km near North Sea have to display a seaonal variation of at least approx. 25 ppm. Fig 89 shows an untypical fluctuation with lows in winter and highs in summer. Nevertheless the graph with 10 days averages show some lunar phase correlation, irregular measurement intervals prevent displying precise monthly cycling. Perhaps Reisets impressive equipment with huge aspirated air volume was rather than for demonstrating accuracy to French Academy of Science. The equipment of Krogh, Lundegardh, Schuftan and Scholander demonstrate that accurate and fast measurements are possible without such large air volumes. According to Martin et al. [106], Kauko [63] and Kleiber [111] absorption efficiency of CO 2 is dependend on concentration of absorption solution, speed of gas flow and design of absorption equipment. Errors risewith large volumina [111]. Incontinous sampling was mentioned above. Reiset led Pettenkofers fast and simple method ad adsurdum beeing not able to analyse diurnal variation. The erronous too low values of Reiset can be seen analysing his data sampled in 4 m to 30 cm above ground in forest and cultivated field. Other authors e.g.Wollny [161] and Lundegardh [52] had found different values: free air red clover Reiset 290 ppm 289,9 ppm (-0,04%) Wollny 2m (- 6%) Lundegardh 317 ppm Rommell* Meinecke* Gut* De Selm (164) 320 ppm (summer) from Duerst ([67], p. 308) barley and other 281,9 (–2,8%) forest 292 (+ 0,7%) oat (up to –40%) 2m ,( up to +70%) treetop ( up to +70% > + 100% ~ + 72% 1, 2 m ( +68%) Table 7 Comparison of measurements in free air and area with vegetation Nervertheless Reiset´s work was mentioned as exemplary and especially accurate throughout contemporary literature e.g. Letts& Blake [53]), Brown&Escombe [145,146], Lundegardh, [52] Callendar [113,119], Keeling [147]. Brown and Escombe actually used Reiset´s absorption apparatus in 1898 modified from 600 l to approx. 200 – 300 liter air. Callendar praised Reiset´s measurements as the most reliable accuracy of which was not achievedlater on despite citing Lundegardh who conducted measurements in southern Sweden using a Pettenkover variant displying lowest error range of +-1% in 1920-1926. Keeling [147] did a detailed discussion of Reiset´s data and denoted them without errors too and certified measurements precision of modern age (1986). This is remarkable because accuracy of Reiset´s Pettenkofer variant was approx. +-3%, that means 9 ppm compared to carbon dioxide 3.5 Verification of further CO2-maximas 1857 and 1876 87 concentration in air at that times of approx. 300 ppm. A diurnal variation of 5 ppm at night is therefore not possible to measure because of long analysing time as Keeling postulated in [147, p. 96]. Fig. 90 CO2 -analysis of air by . Reiset 1872/72 cited by From& Keeling (1986) compared to Reiset´s data out of original paper [144] Fig 90 displays Reiset´s data averaged per month found in [147, p. 96, Fig. 3] (left part) sampled in 1872/73 [144]. Comparison of data extracted out of Reiset´s peper [144] to the data cited by From and Keeling results in poor correlation with lacking july/august 1873 data. From&Keeling´s graph show a seasonal variation which cannot be extracted out of the original Reiset paper. Summary: Data sampled and analysed by v. Gilm 1856/57, Schulze 1863/64, Haesselbarth 1874/75, Farsky 1874/75 and Reiset 1872-1879 were summarized. Despite of a less amount of values prior to 1880 CO 2 data fit well to temperature maxima (IPCC) around 1857 and around 1876. All inspected measurements reflect a monthly cycling corresponding to lunar phase change by displying CO 2 maxima during full moon. With exception of Reiset´s data showing procedural and systematic errors ( in the order of 20 ppm too low) not considered by modern scientists (e.g. Callendar, Keeling) and all consecutive institutions in 20 th century( WMO, IPCC) great majotity of available data show higher CO 2 values as reconstructed out of ice core data. 3.5 Verification of further CO2-maximas 1857 and 1876 3.6 88 Evaluation of the CO2 characteristics before 1857 Evaluation of CO2 measurements prior to 1857 ( see table 4) is difficult because of few data, lacking calibration, comparative measurements and some prooven systematic and procedural errors ( e.g. Thenard, Brunner, Regnault) e.g. using highly absorptive sulfuric acid for drying air. Using of gravimetric ( Thenard, Brunner, Boussingault) and volumetric (Regnault) analysing procedures as open systems including eudiometers which allowed determination of CO2 as a difference of O2 and N2 results in partly inaccurate data. ( see discussion of methods) Additionally no continous sampling was conducted so preventing of accurate tracking diurnal and seasonal variation. Previous review of historic data reveals in contrast to modern published conclusions that carbon dioxide content of air follows temperature and so climate in agreement with known laws of thermodynamics and dissolution. Indication is drawn out of inspection of extended temperture measurements available down to 1740 in northern hemisphere and reliable since approx. 1820 [51]. Modern temperature graphs published by IPCC, Hansen or others present data only since approx. 1880. Here I present the result using approx 17 averages since 1812 (see table 4)showing an error range not beeing able to quantify accurately: Fig. 91 Envelope of CO2 –analysis in air by chemical methods prior to 1857 (see table 4) compared to ice core analysis, showing Tambora volcanic event in 1815, de Saussures measuring series and 3% error range since 1856 (v. Gilm).See linear falling trend up to the late 19 th century. Total 19th century average using 81 yearly averages since 1812 –1900 (data 1880 –1900 not included in Fig. 91) result in 322 ppm in contrast to 285 ppm published by modern climate scientists showing a difference of + 11,5%. Dispite of unknown accuracy prior to 1857 outstanding carefull analysed data by de Saussure (1811-1830) indicate higher level of air content in the beginning if the 19 th century compared to the late decades with lowest levels around 1890 corresponding to low levels in temperature. High temperatures can also be seen in extended temperature measurements compiled by Angell et al.1885 showing a peak around 1825 see fig. 94. Out of 17 reviewed papers dating back to first half of 19th century I will present extracts of the work of two scientists to show characteristics of data analysed prior to 1857. 3.5 Verification of further CO2-maximas 1857 and 1876 89 Fig. 92 Chemical analysis of CO2 in air of Paris by Boussingault 1829-1841. J.B. Boussingault, famous French chemist at university Lyon and Paris conducted several series of measurements of CO2 in air in France between 1833 and 1853 [53, 200]. In early years he determined CO2 content in air at Lyon to 600 –800 ppm [201]. Later on he calculated the amount of carbon dioxide produced in Paris within 24 hours to 2,9 x 106 m3. Between january 1840 and july 1841 he conducted 142 almost daily measurements in Paris using the Brunner apparatus (gravimetric, drying air by sulfuric acid) resulting in an average of 400 ppm ( see fig. 92). He also sampled and analysed air in other localities inclusive rural areas in France not presented here resulting in winter-spring 1839/40 in 365 ppm, his 48 measurements at night in Paris resulted in an average of 420 ppm in comparison to sampling at daytime of 390 ppm which confirmed diurnal variation [199]. In total daily values sampled in his Paris series show strong fluctuations within a 200 –700 ppm range showing local influence of the city, and systematic errors due to weighing and absorption in sulfuric acid and others. Winter maxima of CO 2 content can be seen in Fig. 92. In contrast to the analyses done with Brunner and Regnault equipment since 1832 T. de Saussure used a modified gravimetric procedure since 1800 also Thenard used 1812 for his gas analysis. He tested several variants and had optimized his analyses until 1826 conducting several 100 measurements at Chambeisy (Lake of Geneva) up to 1830 [50]. The latest analyses are most accurate conducted on a well ventilated grassland area, 250 m wide, soil of clay , 16 m above sea level of Lake Geneva at Chambeisy lying 388 m above ocean level. 35 -45 l air was sucked by an air pump through a solution of Baryta Water and the resulting carbonate precipitated by sulfuric acid and then dried and weighed. Absorption was several hours, the absorbing vessel was washed using HCl and combined with absorbing Baryta Water. Data prior to 1826 by de Saussure are not presented here. Out of his measuring series between 1826 to 1830 ( approx 200 data) 66 values were selected concerning the same measuring conditions in time and weather without rain or fog. Precise measuring cinditions are presented in chapter 2.3 [1], methods. He tested in detail influence of humidity (rain, fog, snow and ice also of ice covered ground). Plotted data using 66 selected values is presented in fig. 93. 3.5 Verification of further CO2-maximas 1857 and 1876 90 Fig. 93 Chemical CO2 gas analysis of air by Th. de Saussure 1826-1830 (see table 4) compared to temperature reconstructed by Angell et al. 1985 including volcanic events and monthly cycling. Full moon: 5-11-1827, 6-9, 7-26-1828, 9-26, 10-23, 2-20-1829, 4-19, 6-17, 7-16, 8-14, 10-12, 12-10. Strikingly is the sharp falling graph down to 1830. Some seasonal can variation also be seen. Because of ffew data within measuring period, no calibration against other methods or later measurements a quantifying of errors is difficult. But monthly cycling corresponding to lunar phases and relative low variation since 1828 indicates high quality of data for that times. Yearly average in 1827 was 481 ppm, 1828: 431 ppm, 1829: 385 ppm. Accepting aconstant systematic and procedural error through 3 years of measurements we are able to quantify a decrease of 140 ppm which clearly correspond to the falling temperature at that times according to the northern hemisphere reconstruction by Angell et al [165]. A comparison with Angells temeperaturereconstruction during the whole time of chemical analysis is also interesting because all evaluated CO2 measurements show correlation to temperature and Angells reconstruction date back to 1800. 3.5 Verification of further CO2-maximas 1857 and 1876 91 Fig. 93 Chemical analysis of CO2 of air 1812 to 1861in northern hemisphere (NH) compared to temperature in NH (Angell et al. [165]) since 1800 including volcanic events Angell et al. [165] used data by Jones et al. 1982 and Grovemann and Landsberg 1979 back to 1740. Th temperature graph shows a maximum around 1827 similat than that in 1942. Correlation in contour is relatively good. The eruption of Tambora has produced a guessed emission of 150 km3 matter compared to Pinatubo 1991 15 times more [202]. Sulfate emission is guessed to 200 Million tons compared to Pintubo 30 Million to. This event led to the “year without summer “ in 1816 as one of the coldest years in 200 years [203]. This analysis show that Reiset or Müntz in the late 19th century wrongly critizised de Saussure for his high level data above 400 ppm which fell down dramaticlly later on. 3.7 Monthly cycling of carbon dioxide content according lunar phases Included in most evaluated historic data are the correlation of a quasi monthly cycling of CO 2 in air at a wavelenght of about 28-30 days. Full moon events correlate with a maximum in CO2 in the order of 10-20 ppm in historic papers with high seasonal variation and some ppm with low variation. Modern NDIR measurements also displays this signal in the order of 1 ppm. 3.5 Verification of further CO2-maximas 1857 and 1876 92 Fig. 93 a Monthly CO2 cycling in modern Mauna Loa measurements 2004 according lunar phases. Full moon at maximum. Often there is a disconnection in summer reinstalled in autumn to winter. Causes seem to be the same as explaining tidal forces; Occurrence, amplitude variation spreading over latitudes had to be further investigated. Because of beeing able to find well fitted variations in nearly all historic data it seems to be s strong test for the validity of old data. Especially the pre-1857 era concerning the de Saussure measurement series up to 1830 gets more validity beside seasonal variation and correlation with temperature maximum. So high CO2 levels around 1825 seems to have really existed. 5. Schlussfolgerungen: 93 4. Discussion Data concerning historical gas analysis in air used in this study are researchable in several comprehensive libraries and papers. Year Authors 1900 Letts and Blake [53] 1912 Benedict [51] 1940 Callendar [113] 1951 Effenberger [54] 1952 Stepanova [118] 1956 Slocum [128] 1958 Callendar [119] 1958 Bray [129] 1986 Fraser [149] 1986 Keeling [147 ] 2006 Beck [this study] 1 see references Cited authors and papers with data Total 19 th c. 20thc. 252 252 137 137 13 7 6 56 32 24 229 130 99 33 22 11 30 18 12 49 20 19 6 6 18 18 156 82 74 Notes only 19th century (+) +; focus on O2-determination cited Letts&Blake and Benedict cited Duerst1, Misra1 and Kreutz1 citation as Effenberger cited Duerst and Kreutz no citing of Duerst, Kreutz and Misra cited most important through the centuries +, same as Callendar +, same as Callendar; only chemical determination until 1961 Table 8 Bibliographies and citation of papers Inspecting more than 250 data sets available in literature I selected 156 measurement series since 1812 to 1961 reducing to 138 yearly averages ( approx. 90 000 single measurements) spread over the northern hemisphere to reconstruct the variation of the CO2 content in air during period of chemical determination. In contrast to modern climate science I used 74 analysed in 20th century beside 82 data sets from 19th century too. Analytical chemistry had evolved and optimized since early 1800 up to the 30s of the 20th century so accurate procedures and test equipment were available in form of the titrimetric Pettenkofer or the volumetric Pettersen/Sonden/Haldane or Schuftan gas analysers. Consequently 26 most important data, locations, methods and scientists are evaluated with the help of test criteria to accept or reject quality of data. Comparison with modern NDIR procedure is only possible by inspecting measurements around 1960 (Steinhauser). Results fit perfectly within the error range (3%) of chemical methods. Steinhauser got a yearly average of 325 ppm +-3% (= +-10 ppm) in the city of Vienna in 1957/58 by using chemical Pettenkofer procedure. At the same time Keeling conducted measurements at Mauna Loa volcano in approx. 3000 m height in marine area resulting in a yearly average of 315 ppm +-1-4% [112]. Difference of 10 ppm can also explained by measuring wet air in Vienna and dry air in Mauna Loa and absorbing about 10 ppm in the oceans around Hawai and measuring in 3000m with a little lower CO 2 level. Further criteria are the reproduction of the seasonal and diurnal variation, calibration and intercalibration of different methods and correlation with lunar or solar influences. For selected measurements since 1857 v.Gilm/Pettenkofer an intercalibration was researchable with systematic errors within 3%. Solar and lunar influences were imaged by most of reviewed data sets in contrast to published literature especially by Callendar and Keeling. Between 1800 and 1961, more than 380 technical papers that were published on air gas analysis contained data on atmospheric CO2 concentrations. Callendar [113, 117, 119] Keeling and the IPCC did not provide a thorough evaluation of these papers and the standard chemical methods that they deployed. Rather, they discredited these techniques and data, and rejected most as faulty or highly inaccurate [119, 141,147, 149, 150, 154]. Though they acknowledge the concept of an ‘unpolluted background level’ for CO 2, these authors only examined about 10% of the available literature, asserting from that that only 1% of all previous data could be viewed as accurate (Müntz [71, 152, 153], Reiset [144], Buch [78]. During the 100 years of using the historic equipment and methods most basics of modern knowledge in medicine, biology and physiology had been established taught today in every texbooks of this diciplines. 5. Schlussfolgerungen: 94 The most important are The photosynthetic balance The cell respiration balance Blood gas metabolism Basic Metabilic Rate (BMR) of man and animals and so on. 6 CO2+ 6 H20 C6H12O6 + 6 O2 C6H12O6 +6 O2 -> 6 CO6 + 6 H2O Several Nobel prices had been awarded to involved scientists (Krogh 1923, Warburg 1933), Benedict was aNobel nominee in 1923 and other awards will be assigned to outstanding scieentific work bearing the name of many of the authors mentioned in this study as for example the Schuftan Memorial Prize in Process Design in Chemical Engineering (UK)- and the Pettenkofer-Preis (medicine, D). Other scientists as Lundegardh made revolutionary contributions to the insight of ecology and olant physiology e.g. by the invention of the flame photometer 1929, or the discovery of the cytochromes in 1950, [100]. And without the accurate determination of blood gas levels by the analyser of van Slyke 100 000s of patients were died during last 70 years. Modern greenhouse hypothesis is based on the work of G.S.Callendar and C.D. Keeling, following S. Arrhenius, as latterly popularized by the IPCC . Review of available literature raise the question if these authors have systematically discarded a large number of valid technical papers and older atmospheric CO2 determinations because they did not fit their hypothesis? Obviously they use only a few carefully selected values from the older literature (<1%), invariably choosing results that are consistent with the hypothesis of an induced rise of CO2 in air caused by the burning of fossil fuel. Evidence for lacking evaluation of methods results from the finding that as accurate selected results show systematic errors in the order of at least 20 ppm. Most authors and sources have summarised the historical CO2 determinations by chemical methods incorrectly and promulgated the unjustifiable view that historical methods of analysis were unreliable and produced poor quality results. Starting point of the modern greenhouse hypothesis were the publications of G.S: Callendar who defined the criteria for an accurate CO2 content of air. Acceptable values should not vary more than „10% from the general average of time and region“. Measurements for biological purpose were inacceptable. [113]. Fig. 94 G. Callendars definition of acceptable CO2 values in 1958 [113] Therefore Callendar rejected all data prior to 1870 because of 50-100% differences betweenthe averages of past authors. So natural variation was excluded as a possible cause. The only accepted 19th century authors were Reiset (a), Müntz (b), Letts&Blake (c) and Brown&Escombe (d, see fig 95) certifying the average of these 4 sources of 290 ppm an accuracy of 1%. The only criteria were size of value and weather, no quantitative evaluation of methods, locations, seasonal or diurnal variation and other which had revealed the systematic errors of procedures used by Reiset and Müntz of at least 10%. 5. Schlussfolgerungen: 95 Fig. 95 Callendars “fuel line” [113] containing selected data according to his 10% variation criteria ignoring data by Duerst, Kreutz, Misra, Lockhart and Scholander measuring the 1942 peak. Fig. 95 shows callendars selction of data with a, b, c (19th century) and 1-12 (20th century). He did not correct the 3 month Lets&Blake series in spring 1897 for seasonal variation and mostly rainy days. Brown&Escombe used the erroneous Reiset method and had incontinous data. Used 20th centuries data ar given in fig.96. Fig. 96 Callendars 20th century data [113] containing selected data according to his 10% variation criteria. Callendars citing of Benedict, Lundegardh, Buch, Haldane was erroneous and selectively too low ( see chapter 3.3), e.g. Lundegardh measured from 1920-1926. Carpenter was not able to detect seasonal variation within a year. Recalculating the averages of Buch´s values show higher ppm and must be 5. Schlussfolgerungen: 96 corrected because of lacking data and sea absorption ( see chapter 3.3). The Haldane value of 324 ppm was an average of 153 samples only in august showing low summer levels. Combining with 386 ppm at night it would be 355 ppm and still too low because of lacking winter data. He ignored data measured by Haldane at the coast in august and september with an average of 370 ppm. In the end Callendar did not realize the procedural errors of the analysis conducted by the scandinavian network between 1950 and 60. Because of his attempt to proove his “fuel line” a verification or falsification of a huge amount of available other data (see Stepanova [118]) especially Duerst, Haldane, Kreutz, Misra, Scholander was not undertaken. In total Keeling joined this view of literature and also presumed overall contamination, faulty methods or local industrial influence [147]. Fig. 97 Keelings reproduction of Callendars „fuel line“ with the Reiset CO2 average of 292 ppm fitting in ice core reconstruction of CO2 by Neftel et al.(1985) [147]) fig. 10, p. 102. Actual value without absorption error supplemented as *. 292,4 ppm as the average of Reiset is praised by Keeling as the accuratest at the end of the 19th century, concerning the five mesuring series Callendar had choosed. In other papers Keeling denoted the Pettenkofer method as extremly inaccurate, Reiset´s variant is judged by him as very accurate within 1-2 ppm meaning an accuracy of 0,3 – 0,7% compared to the average of Reiset. According to Stanhill (Climate change, 1984, 6, 409) Keeling himself achieved up to 1976 an accuracy of +-4 ppm with NDIR method because of errors. Lundegardh 1920 measured within 1%, or +- 3 ppm using an optimized automatic apparature. Kauko analysed Pettenkofer method thoroughly in 1932 [63] resulting in a theoretical accuracy of simple Pettenkofer method of +-0,0006 Vol%. d.h. 2% so Keelings indication of 1-2 ppm accuracy for Reiset´s equipment can be contradicted. Nevertheless Keeling postulated that Reiset was the first scientist that presented correct seasonal variation. This honor is due to Th. de Sausure 1830 and later Schultze 1864. The accuracy of Callendars and Keeling evaluation of Reisets work is underlined by the fact that Keeling named Reiset 1993 a Belgian and Dieppe, the location Reiset analysed would be placed at Belgian coast [47]. Callendar confused the surname of Reiset instead of Jules he cited him as Jean Reiset. [113]. Callendar and Keeling also praised the data analysed by Müntz especially because of low CO2 levels down to 0,02% during French expedition to Antarctica 1908-1910. In reviewed literature of Callendar and Keeling there is no precise evaluation of the open, volumetric gas analyser of Muentz researchable. (See 5. Schlussfolgerungen: 97 the above mentioned systematic absorption errors and no seasonal and diurnal variation visible in data by Muentz). Other authors as de Saussure 1826-1830 or Petermann 1884 were cited erroneously and data wer selected to fit. Review of more then 250 papers ( approx. 200 cited here) there is no evalution of teh work of the most accurate measurement series done by W. Kreutz 1939-1941 at Giessen, Germany. Sampling an analysing 120 daily data with an automatic, electrified registration system for weather data led to more than 70 000 CO2 values analysed with similar accuracy Keeling had done 20 years later. [126] showing precise seasonal variation. Reserach on history of CO2 gas analysis revealed that the chemist Keling did probably no study of literature in 1954 when he began his inverstigations. He also did not use the approved Scholander Gasanalyser available at that times because Scholander was also a member of the same institute at that times. Ignoring available knowledge he went to the field and conducted measurements using a self made manometer needing several hours for one value resulting in the same CO 2 concentrations at all location he measured. [47]. Keelings merits are the development of an extremely accurate gas analyser and proof of a rising CO2 content in air since 1958. The merits of Callendar are minor to his selective and speculative postulation of rising CO 2 since 1880 to 1930 by about 20 ppm. His speculative cause with inacceptable criteria for excluding accurate values was burning fossile fuels so he established modern greenhouse thesis. This study prooves contradicting Callenadar that this rise can also be reconstructed by using available data rejected by Callendar and Keeling. Most authors cited in this study from, de Saussure 1826 to Scholander in 1947 had the only aim to measure as accurate as possible local CO2 concentration in air. Review of available literature shows however that the authors in the 19th century denoted the CO 2 content in air as constant as the O2 concentration. In 20th century e.g. Lundegardh discussed in detail diurnal and seasonal variations in available literature since de Saussure and the dependence on weather., vegetation soil respiration and ocean/sea absorption [52]. Because of no indication for a significant enduring natural variation of atmospheric CO 2 concentration seen in his data between 1920 to 26 he did not deal with this issue beside the fact that he knows of Arrhenius speculations on CO2 induced temperature rise. As mentioned above Callendar 1938 and Keeling since 1958 categorical excluded possible enduring natural CO2 variations, denoted it as errors or ignored those data as e.g. the CO 2 maximum in 1940. Most interestingly Fonselius [80] displayed some data forming this 1942 maximum (Duerst, Kreutz and Misra) but ignored quality also cited by Slocum in [128]. 5. Schlussfolgerungen: 98 Fig. 98 Fonselius graph of available CO2 data 1800-1955 including data showing peak in 1942 [80]. Thick grey line is overlayed graph of CO2 analysis by chemical means (this study) showing peak in 1942, Fonselius correctly cited. Encircled are the data Callendar used. Analysing of backgroundlevel in maritime areas to get the actual CO 2 concentration is not the only concept of getting well mixed CO2 data of air. Lundegardh in 1920 still discussed in detail influence of free air by several biotic and abiotic factors and absorption of CO 2 by sea resulting a lower CO2 level in ocean air. So Callendar was not the inventor of free air concept. In fact only desert air was uninfluenced by biota, soil and water absorption only winds may change local concentrations. CO2- reconstructions out of ice cores seem to be inaccurate because not resolving CO 2 peaks presented in this study. In several papers Jaworowski claimed this indication [176, 205] because of producing artefacts by decompressing cores cutting them in small fractions and so resulting in gas leakage by transition of clathrates to bubbles. So further investigations have to be undertaken to elucidate accuracy of ice core proxies. Carefully analysing CO2 data sampled on continental areas also supply accurate values taken into account the severals sources and sinks and measuring under selected conditions. Especially the CO 2windspeed-relation used by F. Massen in [204] enabled to calculate by asymptotic approximation nearbackground level average out of data. Following a testing of the Kreutz data analysed 1939-40 at Giessen (Germany) by the Massen procedure: 5. Schlussfolgerungen: 99 Fig. 99 CO2-windspeed correlation in data by Kreutz september 1939 - january 1940 at Giessen [91] calculating asymptotic background level of 434 ppm by F. Massen [204]; CO 2 and wind data measured in height of 14 m. The CO2-windspeed test by Massen et al. revealed a background level at Giessen 1939/40 of 434,1 ppm in 14 m. Kreutz averages the data to 434 ppm which means 100% agreement. So this is a further indication of reliability of historical data. Analysing the data of Steinhauser 1957/58 by the CO2-windspeed-test revealed the same reliability: Fig. 100 CO2-windspeed correlation in data by Steinhauser 1957 at weather station Hohe Warte Vienna [175] calculating asymptotic background level of 324 ppm by F. Massen [204]; CO 2 5. Schlussfolgerungen: 100 The corresponding early Keeling measurements at Mauna Loa in 1958 show a yearly mean is about 313 ppm. With a latitude correction [204] of 1 ppm we get 314 ppm. Adding a similar correction to the Steinhauser values we get 323 ppm and a difference of 9 ppm. This is well within 3% error range derived for Pettenkofer procedures (Steinhauser used Krogh-Pettenkofer-variant, the same was used by Fonselius and Bischof in Scandinavian network). So we are able to show that historic Pettenkofer process and modern NDIR-procedures fit within systematical error range and therfore old methods are valid. 5. Conclusions Finally let´s summarize established knowledge by historical authors reviewed in this study. During the late 20th century, the hypothesis that the ongoing rise of CO 2 concentration in the atmosphere is a result of fossil fuel burning became the dominant paradigm. To establish this paradigm, and increasingly since then, historical measurements indicating fluctuating CO 2 levels between 300 and more than 400 ppmv have been neglected. A re-evaluation has been undertaken of the historical literature on atmospheric CO2 levels since the introduction of reliable chemical measuring techniques in the early to middle 19 th century. More than 90,000 individual determinations of CO2 levels are reported between 1812 and 1961. The great majority of these determinations were made by skilled investigators using well established laboratory analytical techniques. Data from 138 sources and locations have been combined to produce a yearly average atmospheric CO2 curve for the northern hemisphere. This curve contradicts to a 19th century or preindustrial CO2 level of 294 ppm. By valid chemical and direct measurements the 19th century CO2 level has to revised to 341 ppm, using data since 1857 with 3% error range at least 310 ppm. The historic data agree with the relative linearly rising CO 2 amount since 1880 to 1930 showing typical difference of about 10-20 ppm more on northern hemisphere continental air compared to ice core reconstructed concentrations. The historical data that I have considered to be reliable can, of course, be challenged on the grounds that they represent local measurements only, and are therefore not representative on a global scale. Strong evidence that this is not the case, and that the composite historical CO 2 curve is globally meaningful, comes from the correspondence between the curve and other global phenomena, including both sunspot cycles and the moon phases, the latter presented here probably first time in literature and the average global temperature statistic. Furthermore, that the historical data are reliable in themselves is supported by the credible seasonal, monthly and daily variations that they display, the pattern of which corresponds with modern measurements. By the CO2-windspeed-test it is possible to approximate background level out of measurements on northern continental areas. Inspected historic measurements fit within analysed error level. Further evidence of historic data come from comparing to oxygen level which displays corresponding minima to CO2 maxima. It is indeed surprising that the quality and accuracy of these historic CO 2 measurements has escaped the attention of other researchers. Validity of historic data open discussion on actual ice core- and C-flux models which do not fit to these observations. They have to be revised or rejected because of their basis as a human induced climate change. The reinvestigated historic data fill the gap IPCC has left beetween large scale time periods of hundred thousands of years and short-time CO2 data (diurnal/seasonal). Carbon dioxide do also follow climate in hundreds and decads of years and there are cyclic fluctuations through the centuries. Looking at CO 2 maxima around 1857, 1942 and 2001 shows a remarkable wavelenght as the Gleisberg-cycle of 70 to 80 years. This suggests a falling of CO2 and temperature in near future. 5. Schlussfolgerungen: 1. Trotz hohem fachwissenschaftlichem Stand der Chemietechnik und Bewährung in Medizin und Biologie ignorierten Callendar und Keeling als die neben Arrhenius wichtigsten Begründer der modernen Treibhaustheorie ( IPCC) einen großen Teil der verfügbaren Fachliteratur zu CO 2Messungen - besonders die des 20. Jahrhunderts -und selektierten nur wenige Daten, die Ihr Forschungsziel einer durch Verbrennung fossile Kohlenstoffverbindungen erhöhten CO2Konzentration bestätigen sollten. Weiterhin beurteilten und reproduzierten sie die wenigen historischen chemischen Messungen teilweise falsch und propagierten ein unzutreffendes Bild über deren Qualität, ohne sich mit der Methodik auseinandergesetzt zu haben. Alle modernen Autoren und Meetings ab ca. 1975 bis zum IPCC (UNO), die sich mit historischen Messungen befassten übernahmen diese selektive, ignorante und unwissenschaftliche Vorgehensweise. Dies ist die historisch nachweisbare Basis der moderenen Treibhaustheorie. 5. Schlussfolgerungen: 101 2. Durch sorgfältige Recherche historischer Fachliteratur der Chemie, Medizin, Ernährungslehre, Physiologie und Agarmeteorologie konnte gezeigt werden, dass es vor 1958 extrem präzise Messungen des CO2-Gehaltes der Luft mit unter 3% Genauigkeit gab, deren Ergebnisse im Widerspruch zur veröffentlichten Meinung der Klimatologen stehen. 3. Der Vergleich des CO2-Verlaufs aus chemischer Messung mit dem Temperaturverlauf der Nordhemisphäre zeigt, dass die CO2-Kurve präzise alle Temperatur-Schwankungen abbildet. Es gibt also keine wie in der Literatur (IPCC) dargestellt seit dem industriellen Zeitalter exponentiell gleichmäßig ansteigende CO2-Kurve sondern eine der Temperatur folgende Kurve. 4. Auch die vorindustrielle CO2-Konzentration der Nordhemisphäre im 19. Jh. war genau so schwankend wie die des 20. Jahrhunderts mit einem großen Maximum um 1825 mit vermutlich über 400 ppm, einem kleineren um 1857 von über 350 ppm. Ein konstanter, vorindustrieller CO 2Wert von 285 ppm existiert nicht und resultiert aus selektiver und fehlerhafter Betrachtung weniger, ungeigneter historischer Daten ab 1880. Diese Studie zeigt klar, dass die wissenschaftliche Basis der modernen anthropogenen Treibhaustheorie des aktuellen Klimawandels nicht der Realität entspricht, weil sie durch selektive Wahrnehmung und Ignoranz der Fakten zum Beweis einer spekulativen Theorie der beteiligten Wissenschaftler zustande kam. Die allgemein akzeptierte vorindustrielle CO2-Konzentration von ca. 285 ppm entspricht zwar ungefähr der tatsächlichen um ca. 1885, ist aber nur eine Momentaufnahme eines stark schwankenden, dem Klima folgenden Verlaufs in 2 Jahrhunderten. Die moderne Temperaturkurve des IPCC (18), Abb. 32 die um 1860 beginnt suggeriert am unteren Rand eher abfallende Temperaturen, tatsächlich belegt der historische Datensatz GHCN eine nach unten ansteigende Temperatur. Die heute als Erkenntnisse propagierten Zusammenhänge über die Ursachen des modernen Klimawandels sind letztendlich falsche und voreilige Schlussfolgerungen aus mangelhaften Analysen von Callendar und Keeling wie auch Arrhenius. Callendar analysierte im Übrigen auch die spektralen Absorptionseigenschaften von CO2 basierend auf den Arbeiten von Arrhenius, ohne bemerkt zu haben dass dessen Analysen auf fehlerhaften spektralen Daten beruht. Die Mängel der Arrhenius-Analyse von CO2 als Wärmeabsorber bzw,. –Emittent lässt sich wiederum durch Analyse der historischen Fachliteratur leicht belegen (siehe Hans Erren: Arrhenius was wrong! (173)). 6. Referenzen 102 6. Referenzen 1. Der Wasserplanet, E.G. Beck, http://www.egbeck.de/treibhaus/ 2. IPCC Climate Change 2001: The Scientific Basis, http://www.unep.no/climate/ipcc_tar/wg1/index.htm 3. GEOCARB III: A REVISED MODEL OF ATMOSPHERIC CO2 OVER PHANEROZOIC TIME, ROBERT A. BERNER and ZAVARETH KOTHAVALA American Journal of Science, Vol. 301, February, 2001, P. 182–204 und PALEOCLIMATE: Enhanced: CO2 and Climate Change; Crowley and Berner Science 4 May 2001: 870-872 http://stephenschneider.stanford.edu/Publications/PDF_Papers/CrowleyBernerScience01.pdf 4. J Veizer, Nir J. Shaviv; Celestial driver of Phanerozoic climate? JOURNAL OF THE GEOLOGICAL ASSOCIATION OF CANADA; 2005 March Volume 32 No. 1 http://www.gac.ca/JOURNALS/TOCs.html und http://www.fiz.huji.ac.il/~shaviv/Ice-ages/GSAToday.pdf 5. Paleomap-Project; Scotese, C.R., Bambach, R.K., Barton, C., Van der Voo, R., and Ziegler, A.M., 1979. Paleozoic Base Maps, J. Geology, 87: 217-277. http://www.scotese.com/ 6. Petit et al.; Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399: 429-436 http://cdiac.esd.ornl.gov/trends/co2/vostok.htm 7. Timelag CO2/Temperatur Vostoc Icecore: Fischer, H., Wahlen, M., Smith, J., Mastroianni, D. and Deck B. 1999. Ice core records of atmospheric CO2 around the last three glacial terminations. Science 283: 17121714. und 8. Mudelsee M (2001) The phase relations among atmospheric CO2 content, temperature and global ice volume over the past 420 ka. Quaternary Science Reviews 20:583–589. http://www.unileipzig.de/~meteo/MUDELSEE/publ/pdf/lag.pdf 9. Eric Monnin, Andreas Indermühle et al. Atmospheric CO2 Concentrations over the Last Glacial Termination, 5 JANUARY 2001 VOL 291 SCIENCE http://www.climate.unibe.ch/pdf/monnin01sci.pdf 10. High-Resolution Holocene CO2 -Record from the Taylor Dome ice core (Antarctica), Indermühle, A., T.F. Stocker, H. Fischer, H.J. Smith, F. Joos, M. Wahlen, B. Deck, D. Mastroianni, J. Tschumi, T. Blunier, R. Meyer, B. Stauffer, 1998, Nature, 398, 121-126 http://www.climate.unibe.ch/abstracts/indermuehle99nat.html 11. Barnola J., Raynaud, R., Korotkevich, Y.S. and Lorius, C. 1987. Vostok ice core provides 160,000 year record of atmospheric 18O Nature 329: 408-414. und 12. Genthon, C. et al. 1987. Vostok ice core: climatic response to CO2 and orbital forcing changes over the last climatic cycle. Nature 329:414-418. 13. Neftel, A. et al. 1982. Ice core sample measurements give atmospheric CO2 content during the past 40,000 yr. Nature 295:220-223. 14. Neftel, A., H. Oeschger, J. Schwander, B. Stauffer, and R. Zumbrunn. 1982. Ice core sample measurements give atmospheric CO2 content during the past 40,000 yr. Nature (London) 295:220-223. 6. Referenzen 103 15. Neftel et al.(1985) Neftel, A., E. Moor, H. Oeschger, and B. Stauffer (1985, 2 May). Evidence from polar ice cores for the increase in atmospheric co2 in the past two centuries. Nature 315, 45-47. http://cdiac.ornl.gov/trends/co2/siple.htm 16. Gases in ice cores; Michael Bender, Proc. Natl. Acad. Sci. USA Vol. 94, pp. 8343-8349, August 1997 http://www.pnas.org/cgi/content/full/94/16/8343 17. Etheridge, D. M., Steele, L. P., Langenfelds, R. L., Francey, R. J., Barnola, J.-M. & Morgan, V. I. (1995) J. Geophys. Res. 101, 4115-4128 [ISI]. 18. Climate Change, IPCC, (http://www.ipcc.ch/pub/un/syreng/spm.pdf) 19. S. Rahmstorf, H.J. Schellnhuber, Der Klimawandel, C.H.Beck, Wissen, ISBN 3-406-50866-9, S. 18 20. Keeling, C.D. 1960. The concentration and isotopic abundance of carbon dioxide in the atmosphere. Tellus 12:200-203. 21. THE INFLUENCE OF MAUNA LOA OBSERVATORY ON THE DEVELOPMENT OF ATMOSPHERIC CO2 RESEARCH ; Charles D. Keeling, Scripps Institution of Oceanography; University of California at San Diego http://www.mlo.noaa.gov/HISTORY/PUBLISH/20th%20anniv/co2.htm 22. Atmospheric carbon dioxide record from Mauna Loa C.D. Keeling and T.P. Whorf Carbon Dioxide Research Group, Scripps Institution of Oceanography, University of California, La Jolla, California 92093-0444, U.S.A. http://cdiac.ornl.gov/trends/co2/sio-mlo.htm 23. Exchanges of Atmospheric CO2 and 13CO2 with the Terrestrial Biosphere and Oceans from 1978 to 2000. Global Aspects Charles D. Keeling1, Stephen C. Piper1, Robert B. Bacastow1, Martin Wahlen1, Timothy P. Whorf1, Martin Heimann2, and Harro A. Meijer3 SIO REFERENCE NO. 01-07 (REVISED FROM SIO REFERENCE NO. 00-22) JUNE 2001 http://cdrg.ucsd.edu/pdf_files/gaim_1.pdf 24. WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH No. 143; GLOBAL ATMOSPHERE WATCH MEASUREMENTS GUIDE; 2001/2003 http://www.wmo.ch/web/arep/reports/gaw143.pdf und http://www.wmo.ch/web/arep/reports/gaw148.pdf 25. CMDL 1. OBSERVATORY, METEOROLOGY, AND DATA ; MANAGEMENT OPERATIONS 1.1. MAUNA LOA OBSERVATORY; R. C. Schnell and the MLO Staff http://permanent.access.gpo.gov/websites/www.cmdl.noaa.gov/publications/annrpt23/c hapter1_1.htm 26. Zustandsbericht 2001, Kapitel 3: The Carbon Cycle and Atmospheric Carbon Dioxide CO2 concentration trends and budgets, S. 3 http://www.grida.no/climate/ipcc_tar/wg1/pdf/TAR-03.PDF 27. Seasonal and interannual variations of atmospheric CO2 and climate By MICHAEL D. DETTINGER*1 and MICHAEL GHIL2, T ellus (1998), 50B, 1–24 http://tenaya.ucsd.edu/~dettinge/co2.pdf 28. Carbon Dioxide Information Analysis Center. 2003. Trends Online: A Compendium of Data on Global Change. Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee. http://cdiac.ornl.gov/trends/co2/contents.htm und 29. WMO Global Atmosphere Watch; World Data Center for Greenhouse Gases http://gaw.kishou.go.jp/wdcgg.html 6. Referenzen 104 30. World Data Centre for Greenhouse Gases (WDCGG); http://gaw.kishou.go.jp/wdcgg/printedmatter/sum29/03co2.pdf 31. World Data Centre for Greenhouse Gases (WDCGG); WMO Greenhouse Gas Bulletin http://gaw.kishou.go.jp/wdcgg.html 32. Studies on carbon flux and carbon dioxide concentrations in a forested region in suburban Baltimore ;John Hom et al. 2001; 1USDA Forest Service, Northeast Research Station, Indiana University, Bloomington, IN http://www.beslter.org/products/posters/johntower_2003.pdf 33. SEASONALVARIATION OFAND THE INFLUENCE OF LAND USE ON CARBON AND WATER VAPOUR FLUXES AT THE SWISS CARBOMONT FIELD SITE N. Rogiers et al. ; Labor für Atmosphärenchemie, Paul Scherrer Institut, Villigen; Geographisches Institut, Universität Bern http://www.giub.unibe.ch/~eugster/publications/POSTER/Rogiers.2003.EGS.pdf 34. Quiescent Outgassing of Mauna Loa Volcano 1958-1994 Steven Ryan ; Mauna Loa Observatory; Hilo, Hawaii 1995 http://www.mlo.noaa.gov/HISTORY/PUBLISH/steve/VolcCO2.htm 35. Atmospheric carbon dioxide record from Mauna Loa; C.D. Keeling and T.P. Whorf; Carbon Dioxide Research Group, Scripps Institution of Oceanography, http://cdiac.ornl.gov/trends/co2/sio-mlo.htm 36. Keeling, C.D., R.B. Bacastow, and T.P. Whorf. 1982. Measurements of the concentration of carbon dioxide at Mauna Loa Observatory, Hawaii. In W.C. Clark (ed.), Carbon Dioxide Review: 1982. Oxford University Press, New York. 37. Reference Gas Preparation and Calibration; NOAA Technical Memorandum ERL-14 CMDL/CARBON CYCLE GREENHOUSE GASES GROUP STANDARDS PREPARATION AND STABILITY Duane Kitzis, Conglong Zhao; NOAA 1999 http://www.cmdl.noaa.gov/ccgg/refgases/airstandard.html und 38. Reference Materials For Oceanic CO2 Measurements Andrew G. Dickson; Scripps Institution of Oceanography University of California, San Diego, La Jolla California USA http://www.pmel.noaa.gov/pubs/outstand/feel2331/refmaterials.shtml 39. Calibration of Infra-red CO2 Gas Analyzers 1 G. C. Bate, A. D'Aoust, and D. T. Canvin; aDepartment of Biology, Queen's University, Kingston, Ontario, Canada http://www.pubmedcentral.gov/articlerender.fcgi?artid=396226 40. AEROCARB Messstation Italien , Monte Cimone, Nordapennin; Breitengrad: 44°11'N, 2165 m http://www.aerocarb.cnrs-gif.fr/sites/ifa/monte_cimone/monte_cimone.html 41. Zhao, C.L., P.P. Tans, and K.W. Thoning, A high precision manometric system for absolute calibrations of CO2 in dry air, Journal of Geophysical Research, 102 (D 5), 5885, 1997. 42. Vorlesung „Meereschemische Analytik“, WS 2002/2003; Prof. Dr. Arne Körtzinger http://www.ifm.unikiel.de/fb/fb2/staff/Koertzinger/files/Meereschemische_Analytik/meereschem_analytik_DIC.pdf 43. Methane and Carbon Dioxide Continuous Measurements at Izana GAW Station (Spain) A.J. Gomez-Pelaez, R. Ramos, J. Perez-delaPuerta; Observatorio Atmosferico de Izana, Instituto Nacional de Meteorologia (INM) http://www.cmdl.noaa.gov/ccgg/co2experts/presentations/gomez-pelaez_a_16_p.pdf 44. APPLICATIONS OF THE GV INSTRUMENTS TRACE GAS FOR METHANE AND CARBON DIOXIDE ISOTOPE STUDIES: LONDON DIURNALS AND IRISH WETLAND EMISSIONS Royal Holloway; University of London; Rebecca et al. 6. Referenzen 105 http://www.cmdl.noaa.gov/ccgg/co2experts/presentations/fisher_r_69_p.pdf 45. A High Precision Manometric System for Absolute Calibrations of CO2 Reference Gases. NOAA/ESRL Global Monitoring Division 325 Broadway R/GMD1; Boulder, CO 80305-3328 http://www.cmdl.noaa.gov/ccgg/refgases/manometer.html 46. Climate Science Pioneer: Charles David Keeling Scripps Institution of Oceanography, UC San Diego http://ucsdnews.ucsd.edu/newsrel/science/keelingobit.asp 47. A Brief History of Atmospheric CO2 Measurements and Their Impact on Thoughts about Environmental Change; Dr. Charles D. Keeling Rede anläßlich: Winner of the Second Blue Planet Prize (1993); http://www.af-info.or.jp/eng/honor/bppcl_e/e1993keeling.txt 48. The distribution of CO2 between atmosphere, hydrosphere, and lithosphere; minimal influence from anthropogenic CO2 on the global "Greenhouse Effect". Tom V. Segalstad ; Mineralogical-Geological Museum; University of Oslo http://www.uio.no/~tomvs/esef/esef0.htm 49. A modified Precision Barometer; A. Germann, J. chim. Phys. 1914, S. 2456 50. Théodore. de Saussure; Sur la variation de l´acide de carbonique atmosphérique Annales de Chimie et Physique, 44[1830], S. 5 51. F. G. Benedict (1912). "The composition of the atmosphere," Carnegie Publication, No. 166, Washington. 52. Lundegardh,h (1924): Der Kreislauf der Kohlensäure in der Natur. Fischer, Jena (680) 53. E. Letts and R. Blake, The carbonic anhydride of the atmosphere; Roy. Dublin Soc. Sc.Proc., N. S., Vol.9, 1899-1902; Scientific Proceedings of the Royal Dublin Society 54. E. Effenberger, “Messmethoden zur Bestimmung des C02-Gehaltes der Atmosphare und die Bedeutung derartiger Messungen für die Biometeorologie und Meteorologie. Zweite Ted: Ergebnisse der bisheren C02-Messungen,” Annalen der Meteorologie, Vierter Jahrgang, Heft 10 bis 12, 1951, pp. 417-427. 55. Marco Beretta; Pneumatics vs. “Aerial Medicine”:Salubrity and Respirability of Air at the End of the Eighteenth Century http://ppp.unipv.it/Collana/Pages/Libri/Saggi/Nuova%20Voltiana2_PDF/quattroo.pdf 56. John Dalton ; Experimental Enquiry into the Proportion of the Several Gases or Elastic Fluids, Constituting the Atmosphere Memoirs of the Literary and Philosophical Society of Manchester 1, 244-58 (1805) Read Nov. 12, 1802 Aus Classic Chemistry, Carmen Giunta; Le Moyne College, USA http://web.lemoyne.edu/~GIUNTA/dalton1.html 57. Otto Warburg, Maßanalytische Bestimmung kleiner Kohlensäuremengen Zeitschrift f. Physiol. Chemie, Bd. 61, S. 261, 1909 58. M. v. Pettenkofer; „ Über eine Methode die Kohlensäure in der atmosphärischen Luft zu bestimmen“; Chem. Soc. Journ. Trans. 10 (1858), S. 292 und Journ. Prakt. Chem. 85, 1862, s. 165 59. Pettenkofer und Voit, Zeitschrift für Biologie, 1866, 2, S. 459 60. Pettenkofer, M. (1862). Ann. Chem. Pharm. Suppl. Bd. 2, I. 61. Jens Klemme, 2003; Entwicklung der Ernährungsforschung bei Wiederkäuern im 19. Jahrhundert Tierärztliche Hochschule Hannover http://elib.tiho-hannover.de/dissertations/klemmej_2003.pdf 6. Referenzen 106 62. MARTIN, W. M. and GREEN, J. R. Determination of carbon dioxide in continuous gas streams. Ind. and Eng. Chem., Anal. Ed. 5: 114-118. 1933. 63. Eine genaue Methode zur Bestimmung des CO2-Gehaltes der Luft Y. Kauko, V. Mantere ; Zeitschrift für anorganische und allgemeine Chemie Volume 223, Issue 1, 1935. Pages 33-44 64. Pettenkofers Respirationsapparat ; Briefwechsel von Dr. Eugen Freih. von Gorup-Besanez http://gorup.heim.at/Briefe/Pettenkofer.htm 65. Regnault, V. & Reiset, J. (1849). Ann. Chim. (Phys.), 26 (3), 299. 66. J.A. Reiset, Compt. Rend., T. 88, p.1007, T. 90, p.1144,1457 (1879-1880) 67. U. Duerst, “Neue Forschungen über Verteilung und Analytische Bestimmung der wichtigsten Luftgase als Grundlage für deren hygienische und tierzüchterische Wertung,” Schweizer Archiv fiir Tierheilkunde, vol. 81, No. 7/8, August 1939, pp.305-3 17. 68. WMK Martin, JR Green - Industrial & Engineering Chemistry Analytical Edition, 1933 - pubs.acs.org http://pubs.acs.org/cgi-bin/abstract.cgi/iecac0/1933/5/i02/f-pdf/f_ac50082a020.pdf?sessid=6006l3 69. Abderhalden, Handbuch der biochemischen Arbeitesmethoden, Berlin 1919, S. 480 70. Lundegardh,h (1922): Neue Apparate zur Analyse des Kohlensäuregehalts der Luft, Biochem. Zeitschr. Bd. 131, 1922, S 109 71. Müntz et Aubin, Ann. Chim. Phys. Serie 5, T.26, 1882, p 222 72. Schuftan, P, Chem. Fabrik, Nr. 51 1933 S. 513 73. Uffelmann, J. Luftuntersuchungen, Archiv f. Hygiene, 1888, S. 262 74. Uffelmann, Hygienische Topographie der Stadt Rostock, W. Werthers Verlag 1889,S. 45 75. A. Krogh u. P.B.Rehberg, Biochemische Zeitschrift, 205 (1929) 265 76 Krogh, A. (1906). Skand. Arch. Physiol. 18, 364. 77. August Krogh, A Gas Analysis Apparatus Accurate to 0·001% mainly designed for Respiratory Exchange Work;; Biochem J. 1920 July; 14(3-4): 267–281. 78. E. Buch, “Der Kohlendioxydgehalt der Luft als Indikator der Meteorologischen Luftqualitat,” Geophysica, vol. 3, 1948, pp. 63-79. 79. Bischof, W., 1960: Periodical variations of the atmospheric CO, content in Scandinavia. Tellus, 12:216-226. 80. Fonselius, Stig, et al. (1956). "Carbon Dioxide Variations in the Atmosphere." Tellus 8: 176-83. 81. Saussure, Ann. chim. et phys., T. 2, 1816 [1830], p. 199. 82. Pettersson, 0. & Sonden, K. (1895). Skand. Arch. Physiol. 6, 16. 83. Sonden, K. & Tigerstedt, R. (1895). Skand. Arch. Physiol. 6, I. 84. Haldane, J. S., Methods of Air Analysis, London, 1912. 85. Bazett, J. Biol. Chem. 139 (1): 81, 1941 6. Referenzen 107 86. PAUL G. LEDIG et al.AN ADAPTATION OF THE THERMAL CONDUCTIVITY METHOD TO THE ANALYSIS OF RESPIRATORY GASES' , http://www.pubmedcentral.gov/picrender.fcgi?artid=434685&blobtype=pdf 87. Van Slyke D. D. et al., Manometric analysis of Gas Mixtures, I,II Biol. Chem. 95 (2): S. 509 und 531. http://www.jbc.org/cgi/reprint/95/2/531 88. Biographie D.D. Van Slyke; Biographical Memoirs V.48 (1976); National Academy of Sciences http://fermat.nap.edu/books/0309023491/html/309.html 89. John B. West, A Century of Pulmonary Gas Exchange; American Journal of Respiratory and Critical Care Medicine Vol 169. pp. 897-902, (2004) http://ajrccm.atsjournals.org/cgi/content/full/169/8/897 91. W. Kreutz,“ Kohlensäure Gehalt der unteren Luftschichten in Abhangigkeit von Witterungsfaktoren,” Angewandte Botanik, vol. 2, 1941, pp. 89-117, 92. Paul Schuftan and the early develeopment of gas- adsorption chromatography; Journal of high reslution chromatography, Vol. 8, Issue 10, S 651-658 1985 93. P. Schuftan, “Gasanalyse in der Technik’. S. Hirzel Verlag,. Leipzig. (1931). 94. A. Vaupel, Mitarbeiter von W. Kreutz beim Deutschen Wetterdienst ; persönliche Mitteilung 2006 95. A Century of Chromatography, Gas Analysis in the First 50 Years John V. Hinshaw, Serveron Corp., Hillsboro, Oregon, USA.; LC•GC Europe November 2003 http://www.lcgceurope.com/lcgceurope/data/articlestandard/lcgceurope/442003/74307/article.pdf 96. P. F. Scholander ; ANALYZER FOR ACCURATE ESTIMATION OF RESPIRATORY GASES IN ONE-HALF CUBIC CENTIMETER SAMPLES ; J. Biol. Chem. 1947 167: 235-250 http://www.jbc.org/cgi/reprint/167/1/235 97. P.F. Scholander J. MICRO GASOMETRIC ESTIMATION OF THE BLOOD GASES. IV. CARBON DIOXIDE; Biol. Chem. Scholander and Roughton 148 (3): 573. 98. Procedures for Exercise Physiology Laboratories, NASA TM-1998-104826 http://ston.jsc.nasa.gov/collections/TRS/_techrep/TM-1998-104826.pdf , 1998 99. Regnault & Reiset; Recherches chimiques sur la respiration des animaux. Ann. de chim. et de phys., 3ème série, 26 : 429. 100: A. Larkum; Contributions of Henrik Lundegårdh; Photosynthesis Research 76: 105–110, 2003. Biographie H. Lundegardh: http://www.life.uiuc.edu/govindjee/Part2/09_Larkum.pdf 101. H. Lundegardh, Klima und Boden und ihre Wirkung auf das Pflanzenleben, Jena 1949 102. Thenard, Traité élém. de chimie, 5 edit., vol1, p.303 103. Hlasiwetz, W, Über die Kohlensäurebestimmung der atmosphärischen Luft, Wien akad. Sitzungsbericht e 20, 1856, S. 189 104: v.Gilm, Über die Kohlensäurebstimmung der Luft Sitzungsberichte d. kaiserl. Akademie d. Wissenschaften Volume 24, 1857 105. Machta, Bulletin American Meteorological Society Vol 53, No. 5, May 1972, 106. Keeling C. D., 1958. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas, Geochim Cosmochim Acta. 13: 322-334. 6. Referenzen 108 107. Keeling CD.1961. The concentration and isotopic abundance of carbon dioxide in rural and marine air, Geochim Cosmochim Acta. 24: 277-298. 108. CRAIG, H. 1953: The geochemistry of the stable carbon isotopes. Geochimica et Cosmochimica Acta 3, 2–3: 53–92. 109. Glückauf, Carbon Dioxide Content of amospheric air, Nature Vol 153, 1944 S. 620 110. THOMAS, M. D. Precise automatic apparatus for continuous determination of carbon dioxide in air. Ind. & Eng. Chem., Anal. Ed. 5: 193-198. 1933. 111. Kleiber, Relation between Volume of respiration Chamber…;Industr. and Eng. Chemistry, Vol 5 No 2, S 98 112. G. Stanhill, The Montsouris series of Carbon dioxide Concentration Measurements 1877 –1910, Climatic Change 4 (1982) 221-237) 113. G. S. Callendar, “Variations of the Amount of Carbon Dioxide in Different Air Currents,” Quarterly Journal of the Royal Meteorological Society, vol. 66, No. 287, October 1940, pp. 395-400. 114. Regnault, Annales de Chimie et de Physique, 1852, 3d ser, 36, S. 385 115. National Geophysical Data Center (NGDC). (http://www.ngdc.noaa.gov/ngdc.html) 116. E. LOCKHART, ARNOLD COURT; OXYGEN DEFICIENCY IN ANTARCTIC AIR ; Monthly weather report, Vol 70, No. 5, 1942 117. Callendar, G.S. (1938). "The Artificial Production of Carbon Dioxide and Its Influence on Climate." Quarterly J. Royal Meteorological Society 64: 223-40. 118. Stepanova, Nina A. (1952). "A Selective Annotated Bibliography of Carbon Dioxide in the Atmosphere." Meteorological Abstracts 3: 137-170. 119. Callendar, G.S. (1958). "On the Amount of Carbon Dioxide in the Atmosphere." Tellus 10: 243-48. 120. Bibliographien 118. Stepanova, Nina A. (1952). "A Selective Annotated Bibliography of Carbon Dioxide in the Atmosphere." Meteorological Abstracts 3: 137-170. 51. F. G. Benedict (1912). "The composition of the atmosphere," Carnegie Publication, No. 166, Washington. 53. E. Letts and R. Blake, The carbonic anhydride of the atmosphere; Roy. Dublin Soc. Sc.Proc., N. S., Vol.9, 1899-1902; Scientific Proceedings of the Royal Dublin Society 54. E. Effenberger, “Messmethoden zur Bestimmung des C02-Gehaltes der Atmosphare und die Bedeutung derartiger Messungen für die Biometeorologie und Meteorologie. Zweite Ted: Ergebnisse der bisheren C02-Messungen,” Annalen der Meteorologie, Vierter Jahrgang, Heft 10 bis 12, 1951, pp. 417-427. 121. http://www.penguins51.com/antarctica.htm#The%20Preservation%20of%20East%20Base 122. NASA; PAST U.S. ANTARCTIC EXPEDITIONS http://quest.nasa.gov/antarctica/background/NSF/facts/fact07.html 123. Brief von Dr.Lockhart 1941,von der Antarktis Station nach USA MIT, Massachusetts http://www.south-pole.com/p000148a.htm 124. The national archives: http://www.archives.gov/research/guide-fed-records/groups/126.html 125. U.S. STATIONS AND CAMPS IN ANTARCTICA 6. Referenzen 109 http://quest.arc.nasa.gov/antarctica/background/NSF/facts/fact02.html 126. W. Kreutz, Spezialinstrumente und Einrichtungen der agrarmeteorologischen Forschungsstelle…; Biokl. Beibl. H2, 1939 127. Fonselius, Microdetermination of CO2 in the air, with current Data for Scandinavia, Tellus 7 (1955), pp.259-265. 128. Slocum,G. HAS THE AMOUNT OF CARBON DIOXIDE IN THE ATMOSPHERE CHANGED SIGNIFICANTLY SINCE THE BEGINNING OF THE TWENTIETH CENTURY, Monthly Weather Review: Vol. 83, No. 10, pp. 225–231. 129. Bray,J, An analysis of the possible recent Change in Atmospheric Carbon Dioxide Concentration, Tellus XI (19599, 2S 220 130. Steven Ryan, Quiescent Outgassing of Mauna Loa Volcano 1958-1994, Mauna Loa Hilo, Hawaii Observatory; http://www.mlo.noaa.gov/HISTORY/PUBLISH/steve/VolcCO2.htm 131. 125 Years service to the nation http://www.imdpune.org/125_years_imd/index.pdf 132. Global Volcanism Program, Smithonian; Mt. Erebus http://www.volcano.si.edu/world/volcano.cfm?vnum=190002=&volpage=erupt&VErupt=Y&VSources=Y&VRep=Y&VWeekly=Y 133. Mt. Erebus Volcano Observatory http://www.ees.nmt.edu/Geop/mevo/mevo.html 134. E. LOCKHART ,A.COURT; OXYGEN DEFICIENCY IN ANTARCTIC AIR, monthly weather review, Vol 70, No 5, May 1942 135. Hock et al.; Composition of the ground-level atmosphere at Point Barrow Journal of meteorology, Vol 9, 1952, S. 441 136: Per F. Scholander (1905-1980 ), Biographical Memoirs V.56 (1987) National Academy of Sciences (NAS) 137. Kaplan et. al, Analyses of global sea surface temperature 1856-1991 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 103, NO. C9, PAGES 18,567-18,589, AUGUST 15, 1998 138. M. v. Pettenkofer, Journ. Prakt. Chem. 85, 1862, s. 165 139. Persönliche Mitteilung von Dr.W . Wranik, Institut für Biowissenschaften, Meeresbiologie Rostock 140. Fr. Schultze, Landw. Versuchsstationen, Bd. 9, 1867, S.217; Bd. 10, 1868, S. 515, Bd. 12, 1875 S. 1, Bd. 14, 1871, S. 366 141. P. Hässelbarth undJ. Fittbogen, Beobachtungen über lokale Schwankungen im Kohlensäuregehalt der atmosphärischen Luft, Landw. Jahrbücher, 8, 1879, S. 669 142. F. Farsky, Bestimungen der atmosphärischen Kohlensäure in den Jahren 1874-1875 zu Tabor in Böhmen, Wien, Akadem. Sitzungsberichte, 74, 1877, Abt. 2, S. 67 143. Stadterneuerung Dahme; http://www.dahme.de/html/altstadt/baugrund_3.pdf http://www.dahme.de/html/altstadt/sanierung.php 144. J. Reiset, Recherches sur la proportion de l´acide carbonique dans l´air ; Annales de Chimie (5), 26 1882, S. 144 145. H. Brown& F. Escombe; On the variation in the amount of Carbon Dioxide in the Air of Kew during the Years 1898 –1901; Proc. Roy. Soc., B. 76, 1905, S 118 6. Referenzen 110 146. . H. Brown& F. Escombe; On a new method or the determination of Atmospheric Carbon Dioxide…; ; Proc. Roy. Soc., B. 76, 1905, S 112 147. From, Eric, and Charles D. Keeling (1986). "Reassessment of Late 19th-Century Atmospheric Carbon Dioxide Variations." Tellus 38B: 87-105. 148. Carpenter; Nature 141, 273, 1938 149. Fraser et al., in „The changing Carbon Cycle”, Springer Verlag 1986, S. 66 150. The Pre-1958 Atmospheric Concentration of Carbon Dioxide; EOS Meeting June 26, 1984 S. 415, 151. Wigley, T., The Preindustrial Carbon Dioxide Level; Climate Change 5, 1983 S. 315 -320 152. Müntz& Aubin, Recherches sur les Proportions d´acide carbonique contenues dans l´air, Ann. Chim. Phys. Serie 5, T.26, 1882, S. 222 153. Müntz&Aubin, L´acide carbonique de l´air, La Nature, Paris, 1882 , 2. Jahr, S. 385 http://cnum.cnam.fr/CGI/page.cgi?4KY28.18/399/ 154. WPC-53, WMO –Report of the Meeting in the CO2-Concentrations from pre-industrial Times I.G.Y 1983 155. Siegenthaler, U. 1984, 19th Century Measurements of atmospheric CO2, Comment Clim. Change 6, 409-411 156. J. Uffelmann, Luftuntersuchungen ausgeführt im hygienischen Institute der Universität Rostock, Archiv f. Hygiene, 8, 1888 S. 262 157. J. Uffelmann, Hygienische Topographie der Stadt Rostock, Wilhelm Werthers Verlag Rostock, 1889 158. A. Petermann, Acide carbonique contenu dans l´air atmospherique, Brux. Mem. Cour., T. 47, 189293, 2. Abt. S. 5 159. Homepage Stadt Gembloux, Belgien: http://www.gembloux.com/histoire/abbaye.htm 160. Osborn, Briffa, SchweingruberJones; Annually resolved patterns of summer temperature over the Northern Hemisphere since AD 1400 from a tree-ring-density network, 2004; Climatic Research Unit, University of East Anglia, United Kingdom http://www.cru.uea.ac.uk/~timo/papepages/osborn_summertemppatt_submit2gpc.pdf 161. Wollny, E., Forschungen aus dem gebiete der Agrarkulturphysik, Bd. 8, 1885, S. 405 162. N.E. Selander Bih. K.Sv. Vet. Akad. Handl., 1888 (aus Lundegardh (52) 163. The royal Society of Edinburgh, Fellowships http://www.rse.org.uk/fellowship/fells_indexp2.pdf 164.de Selm, H.R., Carbon Dioxide Gradients in Beech Forest in Central Ohio, Ohio Journal of Science 52 (4) 187, Juli 1952 165. Angell,J. et al., Surface Temperature Changes Following the six Major Volcanic Episodes between 1780 and 1980; Journal. of Climate and Appl. Meteorology; Vol 24, 1985, S. 937 166. Keeling, C.D., Atmospheric Carbon Dioxide in the 19th Century, Science, 202, S. 1109, 1978 167. Maynard LA. Francis Gano Benedict--a biographical sketch (1870-1957); J Nutr. 1969 May;98(1):18. und http://oasis.harvard.edu:10080/oasis/deliver/~med00029 6. Referenzen 111 168. Hallands Väderö; http://hem.passagen.se/vaderon/ 169. Landwirtschaftliche Schule Tabor vor 1900; http://sechtl-vosecek.ucw.cz/cml/dir/school_farm.html 170. CDIAG, GHCN-Datensatz; http://cdiac.esd.ornl.gov/epubs/ndp/ndp041/ndp041.html 171. Jones et al.; Climatic Research Unit 2006; http://www.cru.uea.ac.uk/cru/data/temperature/ 172. Hansen, J.E. et al, NASA GISS Surface Temperature analysis. http://cdiac.ornl.gov/trends/temp/hansen/hansen.html 173. Erren, H. Arrhenius was wrong.; http://members.lycos.nl/ErrenWijlens/co2/arrhrev.htm 174. National Climate Center New Sealand http://www.niwascience.co.nz/ncc/clivar/pastclimate 175. Steinhauser, F. Der Kohlendioxidgehalt der Luft in Wien und seine Abhängigkeit von verschiedenen Faktoren, Berichte des deutschen Wetterdienstes, Nr. 51, S 54, 1958 176. Jaworowski, Z., Ancient atmosphere - validity of ice records. Environ. Sci. & Pollut. Res., 1994. 1(3): p. 161-171. ######## Ergänzung 175. Rheinau, E. Praktische Kohlensäuredüngung in Gärtnerei und Landwirtschaft, Springer Verlag Berlin, 1927 176. Riedel, Friedrich; Deutsche Patentschrift Nr. 605333, 18.10.1934; Gasuntersuchungsapparat 177. Grünwald, T., Langfristige Beobachtung von Kohlendioxidflüssen mittels Eddy-Kovarianz-Technik über einem Altfichtenbestand im Tharandter Wald; Tharandter Klimaprotokolle, Technische Universität Dresden, Band 7, März 2003 178. Einecke, G., Der Bergbau und Hüttenbetrieb im Lahn- und Dollgebiet und in Oberhessen, Verlag berg- und hüttenmännischer Verein zu Wetzlar, 1932 179. Georg, R., Haus, R. Porezag, K., Eisenerzbergbau in Hessen , Wetzlar 1986 180. Kauko, Y., Yli-Uotila,T., Zur Kenntnis der absoluten Kohlensäure der Luft, Meteorologische Zeitschrift, Band 54,1937, S. 30-33 181. Spring, W., Roland, L. Untersuchungen über den Kohelnsäuregehalt der Luft; Chemisches Centralblatt Nr. 6, 10.2.1886, 3. Folge 17. Jahrgang and Mémoires couronnés par l´ Academie royal de Belgique, 37, 1885, p. 3 182. Archiv der Stadt Gießen; persönliche Mitteilung 7-10/2006/, Leiter Dr. Brake 183. HENNINGER, S., W. KUTTLER (2004): Mobile measurements of carbon dioxide within the urban canopy layer of Essen, Germany. In: Proc. Fifth Symposium of the Urban Environment, 23.-26. August 2004, Vancouver, Canada, American Meteorological Society, pp. J 12.3. 184. Brunner, M., Annales de Chimie et de Physique, ser. 3, 1841,3, S. 305 185. Brunner., M. Journ. De Pharm. 18, (1832), p.350 186. Treadwell, F.P. Kurzes Lehrbuch der analytischen Chemie, II. Band; Wien 1949, S. 511 187. Corwin et al., Advantages of Butyl Rubber in Organic Analysis; Analytical Chemistry, 1948, S. 1116 188. L. Halleux et al., L'évolution des sciences et des techniques en Wallonie , Gouvernement Wallon, Namur, 1995 6. Referenzen 112 http://www.wallonie-en-ligne.net/1995_Wallonie_Atouts-References/1995_ch09-2_Halleux-R_Bernes-AC_Etienne-L.htm 189. S. Ross, Spring, Walthère Victor. C. C. Gillispie, ed., Dictionary of Scientific Biography, Vol. XII (New York: Charles Scribner's Sons, 1975). 190. Fabrice Muller; La région liégeoise vue du ciel, 2005; http://www.fabricemuller.be/liege/sat/zoom/region/region.html 191. NASA Eclipse Homepage, moon phases; http://sunearth.gsfc.nasa.gov/eclipse/phase/phasecat.html 192, Haldane , J.B.S. Carbon dioxide content in atmospheric air; Nature, 4,4, p. 575, 1936 193. T. Illomets, Chemistry and Chemistry-Related Sciences at Tartu (Dortpat) University in 1802 – 1919 http://mega.chem.ut.ee/obki/keemia1/TU1802_1919.htm 194. J. Heimann, „ Der Kohlensäuregehalt der Luft in Dorpat bestimmt in den Monaten Juni bis September 1888“, Inaug. Dissert. Dorpat 1888; see Letts&Blake [53]. 195. E. v. Frey, „ Der Kohlensäuregehalt der Luft in Dorpat bestimmt in den Monaten September 1888 bis Januar 1889“, Inaug. Dissert. Dorpat 1889; see Letts&Blake [53]. 196. V. Feldt, „ Der Kohlensäuregehalt der Luft in Dorpat bestimmt in den Monaten Februar bis Mai 1887“, Inaug. Dissert. Dorpat 1887; see Letts&Blake [53]. 197. H. Heine, “Ueber die Absorption der Waerme durch Gase und eine darauf beruhende Methode zur Bestimmung des Kohlensäuregehaltes der atmosphärischen Luft“., Wiedemanns Annalen, 16 (1882), p. 441 198. IUPAC NIST Solubility database; http://srdata.nist.gov/solubility/sol_detail.asp?sys_ID=62_79 199. M. Boussignault. “Recheches sur la Quantite d´acide Carbonique contenue dans l´air de la ville de Paris", Annales de chimie et de physique, serie 3, tome 10, 1884, p. 456 1844 (3e série / Tome 10). 200. J. B. Boussingault and Platinum; D. Reidel Publishing Company, Dordrecht and London, 1984, 280 pages, L35.50 http://www.platinummetalsreview.com/pdf/pmr-v28-i4-189-189.pdf 201. J.B. Boussingault; "Recherches sur la composition de l´atmosphere"Comptes rendus hebdomadaires des séances de l'Académie des sciences.1, 1835, p. 36 202. Stothers, R.B., 1984, The Great Tambora Eruption of 1815 and Its Aftermath: Science, v. 224, 1191-1198 203 W. Rammacher, März 2004; http://www.winterplanet.de/Sommer1816/Anhang.html 204. F. Massen et al. Seasonal and Diurnal CO2 Patterns at Diekirch, LU 2003 – 2005; Physics Lab and meteoLCD, Lycée Classique de Diekirch http://meteo.lcd.lu/papers/co2_patterns/co2_patterns.html 205. Z. Jaworowski, T.V. Segalstad and N. Ono, "Do glaciers tell a true atmospheric CO2 story?" The Science of the Total Environment, 114 (1992): 227-284. 206. R.A. Smith, Air and Rain, The beginnings of a chemical climatology (1872); London : Longmans, Green, and co. http://www.archive.org/details/airrainbeginning00smitiala 207. Gut, R. , le Gaz carbonique de lß atmosphere forrestiere, Ders. Journ. Frorest. Suisse 9/10,11,12,1929 6. Referenzen 113 Alphabetisches Literaturverzeichnis: (RN = Reihenfolge der Nennung) RN Quelle 131. 125 Years service to the nation http://www.imdpune.org/125_years_imd/index.pdf 69. Abderhalden, Handbuch der biochemischen Arbeitesmethoden, Berlin 1919, S. 480 40. AEROCARB Messstation Italien , Monte Cimone, Nordapennin; Breitengrad: 44°11'N, 2165 m http://www.aerocarb.cnrs-gif.fr/sites/ifa/monte_cimone/monte_cimone.html 165. Angell,J. et al., Surface Temperature Changes Following the six Major Volcanic Episodes between 1780 and 1980; Journal. of Climate and Appl. Meteorology; Vol 24, 1985, S. 937 11. Barnola J., Raynaud, R., Korotkevich, Y.S. and Lorius, C. 1987. Vostok ice core provides 160,000 year record of atmospheric 18O Nature 329: 408-414. und 39. Bate, G. C., D'Aoust, A. and Canvin; D. T., Calibration of Infra-red CO2 Gas Analyzers Department of Biology, Queen's University, Kingston, Ontario, Canada http://www.pubmedcentral.gov/articlerender.fcgi?artid=396226 85. 1. 16. 51. 55. Bazett, J. Biol. Chem. 139 (1): 81, 1941 Beck E.G., Der Wasserplanet, http://www.egbeck.de/treibhaus/ Bender, Michael, Gases in ice cores; Proc. Natl. Acad. Sci. USA Vol. 94, pp. 8343-8349, August 1997 http://www.pnas.org/cgi/content/full/94/16/8343 Benedict F. G., (1912). "The composition of the atmosphere," Carnegie Publication, No. 166, Washington. Beretta, Marco; Pneumatics vs. "Aerial Medicine":Salubrity and Respirability of Air at the End of the Eighteenth Century http://ppp.unipv.it/Collana/Pages/Libri/Saggi/Nuova%20Voltiana2_PDF/quattroo.pdf 3. BERNER, ROBERT A., and ZAVARETH KOTHAVALA GEOCARB III: A REVISED MODEL OF ATMOSPHERIC CO2 OVER PHANEROZOIC TIME, 120. Bibliographien Stepanova, Nina A. (1952). "A Selective Annotated Bibliography of Carbon Dioxide in the Atmosphere." Meteorological Abstracts 3: 137-170. Benedict, F. G. (1912). "The composition of the atmosphere," Carnegie Publication, No. 166, Washington. Letts, E. and Blake, R., The carbonic anhydride of the atmosphere; Roy. Dublin Soc. Sc.Proc., N. S., Vol.9, 1899-1902; Scientific Proceedings of the Royal Dublin Society Effenberger, E., "Messmethoden zur Bestimmung des C02-Gehaltes der Bedeutung derartiger Messungen für die Atmosphare und die Biometeorologie und Meteorologie. Zweite Ted: Ergebnisse der bisheren C02-Messungen," Annalen der Meteorologie, Vierter Jahrgang, Heft 10 bis 12, 1951, pp. 417-427. 88. Biographie D.D. Van Slyke; Biographical Memoirs V.48 (1976); National Academy of Sciences http://fermat.nap.edu/books/0309023491/html/309.html 79. Bischof, W., 1960: Periodical variations of the atmospheric CO, content in Scandinavia. Tellus, 12:216-226. 129. Bray,J, An analysis of the possible recent Change in Atmospheric Carbon Dioxide Concentration, Tellus XI (19599, 2S 220 123. Brief von Dr.Lockhart 1941,von der Antarktis Station nach USA MIT, Massachusetts http://www.south-pole.com/p000148a.htm 146. Brown, H. & Escombe, F.; On a new method or the determination of Atmospheric Carbon Dioxide...; ; Proc. Roy. Soc., B. 76, 1905, S. 112 6. Referenzen 114 145. Brown, H. & Escombe, F.; On the variation in the amount of Carbon Dioxide in the Air of Kew during the Years 1898 -1901; Proc. Roy. Soc., B. 76, 1905, S 118 78. Buch, E., "Der Kohlendioxydgehalt der Luft als Indikator der Meteorologischen Luftqualitat," Geophysica, vol. 3, 1948, pp. 63-79. 113. Callendar, G. S., "Variations of the Amount of Carbon Dioxide in Different Air Currents," Quarterly Journal of the Royal Meteorological Society, vol. 66, No. 287, October 1940, pp. 395-400. 117. Callendar, G.S. (1938). "The Artificial Production of Carbon Dioxide and Its Influence on Climate." Quarterly J. Royal Meteorological Society 64: 223-40. 119. Callendar, G.S. (1958). "On the Amount of Carbon Dioxide in the Atmosphere." Tellus 10: 243-48. 28. Carbon Dioxide Information Analysis Center. 2003. Trends Online: A Compendium of Data on Global Change. Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee. http://cdiac.ornl.gov/trends/co2/contents.htm und 148. Carpenter; Nature 141, 273, 1938 170. CDIAG, GHCN-Datensatz; http://cdiac.esd.ornl.gov/epubs/ndp/ndp041/ndp041.html 18. Climate Change, IPCC, (http://www.ipcc.ch/pub/un/syreng/spm.pdf) 46. Climate Science Pioneer: Charles David Keeling Scripps Institution of Oceanography, UC San Diego http://ucsdnews.ucsd.edu/newsrel/science/keelingobit.asp 108. CRAIG, H. 1953: The geochemistry of the stable carbon isotopes. Geochimica et Cosmochimica Acta 3, 2-3: 53-92. 134. Crowley and Berner, American Journal of Science, Vol. 301, February, 2001, P. 182-204 und PALEOCLIMATE: Enhanced: CO2 and Climate Change; Science 4 May 2001: 870-872 http://stephenschneider.stanford.edu/Publications/PDF_Papers/CrowleyBerner Science01.pdf 56. Dalton, John; Experimental Enquiry into the Proportion of the Several Gases or Elastic Fluids, Constituting the Atmosphere Memoirs of the Literary and Philosophical Society of Manchester 1, 244-58 (1805) Read Nov. 12, 1802 Aus Classic Chemistry, Carmen Giunta; Le Moyne College, USA http://web.lemoyne.edu/~GIUNTA/dalton1.html 164. de Selm, H.R., Carbon Dioxide Gradients in Beech Forest in Central Ohio, Ohio Journal of Science 52 (4) 187, Juli 1952 27. DETTINGER* MICHAEL D. and MICHAEL GHIL2, Seasonal and interannual variations of atmospheric CO2 and climate T ellus (1998), 50B, 1-24 http://tenaya.ucsd.edu/~dettinge/co2.pdf 38. 67. 54. Dickson, Andrew, G. Reference Materials For Oceanic CO2 Measurements Scripps Institution of Oceanography University of California, San Diego, La Jolla California USA http://www.pmel.noaa.gov/pubs/outstand/feel2331/refmaterials.shtml Duerst, U., "Neue Forschungen über Verteilung und Analytische Bestimmung der wichtigsten Luftgase als Grundlage für deren hygienische und . tierzüchterische Wertung," Schweizer Archiv für Tierheilkunde, vol 81, No. 7/8, August 1939, pp.305-3 17. Effenberger, E., Messmethoden zur Bestimmung des C02-Gehaltes der Atmosphare und die Bedeutung derartiger Messungen für die Biometeorologie und Meteorologie. Zweite Ted: Ergebnisse der bisherigen C02-Messungen," Annalen der Meteorologie, Vierter Jahrgang, Heft 10 bis 12, 1951, pp. 417-427. 173. Erren, H. Arrhenius was wrong.; http://members.lycos.nl/ErrenWijlens/co2/arrhrev.htm 17. Etheridge, D. M., Steele, L. P., Langenfelds, R. L., Francey, R. J., Barnola, J.-M. & Morgan, V. I. (1995) J. Geophys. Res. 101, 4115-4128 [ISI]. 142. Farsky, F., Bestimungen der atmosphärischen Kohlensäure in den Jahren 1874-1875 zu Tabor in Böhmen, Wien, Akadem. Sitzungsberichte, 74, 1877, Abt. 2, S. 67 7. Fischer, H., Wahlen, M., Smith, J., Mastroianni, D. and Deck B. Timelag CO2/Temperatur Vostoc Icecore: 1999. Ice core records of atmospheric CO2 around the last three glacial terminations. Science 283: 1712-1714. und 127. Fonselius, Microdetermination of CO2 in the air, with current Data for Scandinavia, Tellus 7 (1955), 6. Referenzen 115 pp.259-265.. 80. Fonselius, Stig, et al. (1956). "Carbon Dioxide Variations in the Atmosphere." Tellus 8: 176-83. 149. Fraser et al., in "The changing Carbon Cycle", Springer Verlag 1986, S. 66 147. From, Eric, and Charles D. Keeling (1986). "Reassessment of Late 19th- Century Atmospheric Carbon Dioxide Variations." Tellus 38B: 87-105. 12. Genthon, C. et al. 1987. Vostok ice core: climatic response to CO2 and orbital forcing changes over the last climatic cycle. Nature 329:414-418. 49. Germann, A., A modified Precision Barometer;, J. chim. Phys. 1914, S. 2456 104. Gilm v., Über die Kohlensäurebstimmung der Luft Sitzungsberichte d. kaiserl. Akademie d. Wissenschaften Volume 24, 1857 132. Global Volcanism Program, Smithonian; Mt. Erebus http://www.volcano.si.edu/world/volcano.cfm?vnum=190002=&volpage=erupt&VErupt=Y&VSources=Y&VRep=Y&VWeekly=Y 109. Glückauf, Carbon Dioxide Content of amospheric air, Nature Vol 153, 1944 S. 620 43. Gomez-Pelaez, A.J. , Ramos, R., Perez-delaPuerta J., Methane and Carbon Dioxide Continuous Measurements at Izana GAW Station (Spain) bservatorio Atmosferico de Izana, Instituto Nacional de Meteorologia (INM) http://www.cmdl.noaa.gov/ccgg/co2experts/presentations/gomez- pelaez_a_16_p.pdf 84. Haldane, J. S., Methods of Air Analysis, London, 1912. 168. Hallands Väderö; http://hem.passagen.se/vaderon/ 172. Hansen, J.E. et al, NASA GISS Surface Temperature analysis. http://cdiac.ornl.gov/trends/temp/hansen/hansen.html 141. Hässelbarth, P. und Fittbogen, J., Beobachtungen über lokale Schwankungen im Kohlensäuregehalt der atmosphärischen Luft, Landw. Jahrbücher, 8, 1879, S. 669 45. High Precision Manometric System for Absolute Calibrations of CO2 Reference Gases. OAA/ESRL Global Monitoring Division 325 Broadway R/GMD1; Boulder, CO 80305-3328 http://www.cmdl.noaa.gov/ccgg/refgases/manometer.html 95. Hinshaw , John V., A Century of Chromatography, Gas Analysis in the First 50 Years Serveron Corp., Hillsboro, Oregon, USA.; LC•GC Europe November 2003 http://www.lcgceurope.com/lcgceurope/data/articlestandard/lcgceurope/442003/74307/article.pdf 103. Hlasiwetz, W, Über die Kohlensäurebestimmung der atmosphärischen Luft, Wien akad. Sitzungsbericht e 20, 1856, S. 189 135. Hock et al.; Composition of the ground-level atmosphere at Point Barrow Journal of meteorology, Vol 9, 1952, S. 441 159. Homepage Stadt Gembloux, Belgien: http://www.gembloux.com/histoire/abbaye.htm 121. http://www.penguins51.com/antarctica.htm#The%20Preservation%20of%20East%20 Base 2. IPCC Climate Change 2001: The Scientific Basis, http://www.unep.no/climate/ipcc_tar/wg1/index.htm 176. Jaworowski, Z., Ancient atmosphere - validity of ice records. Environ. Sci. & Pollut. Res., 1994. 1(3): p. 161-171. 171. Jones et al.; Climatic Research Unit 2006; http://www.cru.uea.ac.uk/cru/data/temperature/ 137. Kaplan et. al, Analyses of global sea surface temperature 1856-1991 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 103, NO. C9, PAGES 18,567-18,589, AUGUST 15, 1998 63. Kauko, Y., Mantere V.,Eine genaue Methode zur Bestimmung des CO2-Gehaltes der Luft Zeitschrift für anorganische und allgemeine Chemie Volume 223, Issue 1, 1935. Pages 33-44 35. Keeling , C.D. and Whorf; T.P. Atmospheric carbon dioxide record from Mauna Loa; Carbon Dioxide Research Group, Scripps Institution of Oceanography, http://cdiac.ornl.gov/trends/co2/sio-mlo.htm 47. Keeling , Charles D., A Brief History of Atmospheric CO2 Measurements and Their Impact on Thoughts about Environmental Change; Rede anläßlich: Winner of the Second Blue Planet Prize (1993); http://www.af-info.or.jp/eng/honor/bppcl_e/e1993keeling.txt 6. Referenzen 22. 116 Keeling . C.D. and T.P. Whorf Atmospheric carbon dioxide record from Mauna Loa Carbon Dioxide Research Group, Scripps Institution of Oceanography, University of California, La Jolla, California 92093-0444, U.S.A. http://cdiac.ornl.gov/trends/co2/sio-mlo.htm 106. Keeling C. D., 1958. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas, Geochim Cosmochim Acta. 13: 322-334. 23. Keeling C. D., Stephen C. Pipe1, Robert B. Bacastow, Martin Wahlen, Timothy P. Whorf, Martin Heimann, and Harro A. Meijer Exchanges of Atmospheric CO2 and 13CO2 with the Terrestrial Biosphere and Oceans from 1978 to 2000. Global Aspects SIO REFERENCE NO. 01-07 (REVISED FROM SIO REFERENCE NO. 00-22) JUNE 2001 http://cdrg.ucsd.edu/pdf_files/gaim_1.pdf 107. Keeling CD.1961. The concentration and isotopic abundance of carbon dioxide in rural and marine air, Geochim Cosmochim Acta. 24: 277-298. 21. Keeling Charles D., THE INFLUENCE OF MAUNA LOA OBSERVATORY ON THE DEVELOPMENT OF ATMOSPHERIC CO2 RESEARCH ; Scripps Institution of Oceanography; University of California at San Diego http://www.mlo.noaa.gov/HISTORY/PUBLISH/20th%20anniv/co2.htm 20. Keeling, C.D. 1960. The concentration and isotopic abundance of carbon dioxide in the atmosphere. Tellus 12:200-203. 166. Keeling, C.D., Atmospheric Carbon Dioxide in the 19th Century, Science, 202, S. 1109, 1978 36. Keeling, C.D., R.B. Bacastow, and T.P. Whorf. 1982. Measurements of the concentration of carbon dioxide at Mauna Loa Observatory, Hawaii. In W.C. Oxford University Press, New York. Clark (ed.), Carbon Dioxide Review: 1982. 37. Kitzis, Duane, Zhao, Conglong Reference Gas Preparation and Calibration; NOAA Technical Memorandum ERL-14 CMDL/CARBON CYCLE GREENHOUSE GASES GROUP STANDARDS PREPARATION AND STABILITY; NOAA 1999 http://www.cmdl.noaa.gov/ccgg/refgases/airstandard.html und 111. Kleiber, Relation between Volume of respiration Chamber...;Industr. and Eng. Chemistry, Vol 5 No 2, S 98 61. Klemme, Jens, 2003; Entwicklung der Ernährungsforschung bei Wiederkäuern im 19. Jahrhundert Tierärztliche Hochschule Hannover http://elib.tiho-hannover.de/dissertations/klemmej_2003.pdf 42. Körtzinger, Arne ,Vorlesung "Meereschemische Analytik", WS 2002/2003; http://www.ifm.uni- kiel.de/fb/fb2/staff/Koertzinger/files/Meereschemische_Analytik/meereschem_ analytik_DIC.pdf 126. Kreutz, W., Spezialinstrumente und Einrichtungen der agrarmeteorologischen Forschungsstelle...; Biokl. Beibl. H2, 1939 91. Kreutz, W.," Kohlensäure Gehalt der unteren Luftschichten in Abhangigkeit von Witterungsfaktoren," Angewandte Botanik, vol. 2, 1941, pp. 89-117, 76 Krogh, A. (1906). Skand. Arch. Physiol. 18, 364. 75. Krogh, A. u..Rehberg ,P.B, Biochemische Zeitschrift, 205 (1929) 265 77. Krogh, A., A Gas Analysis Apparatus Accurate to 0·001% mainly designed for Respiratory Exchange Work;; Biochem J. 1920 July; 14(3-4): 267-281. 169. Landwirtschaftliche Schule Tabor vor 1900; http://sechtl-vosecek.ucw.cz/cml/dir/school_farm.html 100: Larkum; A., Contributions of Henrik Lundegårdh; Photosynthesis Research 76: 105-110, 2003. Biographie H. Lundegardh: http://www.life.uiuc.edu/govindjee/Part2/09_Larkum.pdf 86. LEDIG, PAUL G. et al.AN ADAPTATION OF THE THERMAL CONDUCTIVITY METHOD TO THE ANALYSIS OF RESPIRATORY GASES' , http://www.pubmedcentral.gov/picrender.fcgi?artid=434685&blobtype=pdf 53. Letts, E. and Blake R., The carbonic anhydride of the atmosphere; Roy. Dublin Soc. Sc.Proc., N. S., Vol.9, 1899-1902; Scientific Proceedings of the Royal Dublin Society 116. LOCKHART, E., COURT, ARNOLD; OXYGEN DEFICIENCY IN ANTARCTIC AIR ; Monthly weather report, Vol 70, No. 5, 1942 70. Lundegardh, H (1922): Neue Apparate zur Analyse des Kohlensäuregehalts der Zeitschr. Bd. 131, 1922, S 109 Luft, Biochem. 6. Referenzen 101. 52. 105. 62. 68. 117 Lundegardh, H.,Klima und Boden und ihre Wirkung auf das Pflanzenleben, Jena 1949 Lundegardh,H., (1924): Der Kreislauf der Kohlensäure in der Natur. Fischer, Jena (680) Machta, Bulletin American Meteorological Society Vol 53, No. 5, May 1972, MARTIN, W. M. and GREEN, J. R. Determination of carbon dioxide in continuous gas streams. Ind. and Eng. Chem., Anal. Ed. 5: 114-118. 1933. Martin, WMK, Green, JR,- Industrial & Engineering Chemistry Analytical Edition, 1933 - pubs.acs.org http://pubs.acs.org/cgi-bin/abstract.cgi/iecac0/1933/5/i02/f- pdf/f_ac50082a020.pdf?sessid=6006l3 167. Maynard, LA. Francis Gano Benedict--a biographical sketch (1870-1957); J. Nutr. 1969 May;98(1):18. und http://oasis.harvard.edu:10080/oasis/deliver/~med00029 9. Monnin, Eric, Andreas Indermühle et al. Atmospheric CO2 Concentrations over the Last Glacial Termination, 5 JANUARY 2001 VOL 291 SCIENCE http://www.climate.unibe.ch/pdf/monnin01sci.pdf 133. Mt. Erebus Volcano Observatory http://www.ees.nmt.edu/Geop/mevo/mevo.html 8. Mudelsee, M., (2001) The phase relations among atmospheric CO2 content, temperature and global ice volume over the past 420 ka. Quaternary Science Reviews 20:583-589. http://www.unileipzig.de/~meteo/MUDELSEE/publ/pdf/lag.pdf 71. Müntz et Aubin, Ann. Chim. Phys. Serie 5, T.26, 1882, p 222 152. Müntz& Aubin, Recherches sur les Proportions d´acide carbonique contenues dans l´air, Ann. Chim. Phys. Serie 5, T.26, 1882, S. 222 153. Müntz&Aubin, L´acide carbonique de l´air, La Nature, Paris, 1882 , 2. Jahr, S. 385 http://cnum.cnam.fr/CGI/page.cgi?4KY28.18/399/ 122. NASA; PAST U.S. ANTARCTIC EXPEDITIONS http://quest.nasa.gov/antarctica/background/NSF/facts/fact07.html 174. National Climate Center New Sealand http://www.niwascience.co.nz/ncc/clivar/pastclimate 115. National Geophysical Data Center (NGDC). (http://www.ngdc.noaa.gov/ngdc.html) 15. Neftel et al.(1985) Neftel, A., E. Moor, H. Oeschger, and B. Stauffer (1985, 2 May). Evidence from polar ice cores for the increase in atmospheric co2 in the past two centuries. Nature 315, 45-47. http://cdiac.ornl.gov/trends/co2/siple.htm 13. Neftel, A. et al. 1982. Ice core sample measurements give atmospheric CO2 content during the past 40,000 yr. Nature 295:220-223. 14. Neftel, A., H. Oeschger, J. Schwander, B. Stauffer, and R. Zumbrunn. 1982. Ice core sample measurements give atmospheric CO2 content during the past 40,000 yr. Nature (London) 295:220223. 160. Osborn, Briffa, Schweingruber, Jones; Annually resolved patterns of summer temperature over the Northern Hemisphere since AD 1400 from a tree-ring- density network, 2004; Climatic Research Unit, University of East Anglia, United Kingdom http://www.cru.uea.ac.uk/~timo/papepages/osborn_summertemppatt_submit2gpc.pdf 139. Persönliche Mitteilung von Dr.W . Wranik, Institut für Biowissenschaften, Meeresbiologie Rostock 158. Petermann, A., Acide carbonique contenu dans l´air atmospherique, Brux. Mem. Cour., T. 47, 189293, 2. Abt. S. 5 6. Petit et al.; Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399: 429-436 http://cdiac.esd.ornl.gov/trends/co2/vostok.htm 59. 60. 138. 58. 64. 82. Pettenkofer und Voit, Zeitschrift für Biologie, 1866, 2, S. 459 Pettenkofer, M. (1862). Ann. Chem. Pharm. Suppl. Bd. 2, I. Pettenkofer, M. v., Journ. Prakt. Chem. 85, 1862, s. 165 Pettenkofer, M. v.; " Über eine Methode die Kohlensäure in der atmosphärischen Luft zu bestimmen"; Chem. Soc. Journ. Trans. 10 (1858), S. 292 und Journ. Prakt. Chem. 85, 1862, s. 165 Pettenkofers Respirationsapparat ; Briefwechsel von Dr. Eugen Freih. von Gorup-Besanez http://gorup.heim.at/Briefe/Pettenkofer.htm Pettersson, 0. & Sonden, K. (1895). Skand. Arch. Physiol. 6, 16. 6. Referenzen 98. 118 Procedures for Exercise Physiology Laboratories, NASA TM-1998-104826 , 1998 http://ston.jsc.nasa.gov/collections/TRS/_techrep/TM-1998-104826.pdf 19. Rahmstorf, S., Schellnhuber, H.J., Der Klimawandel, C.H.Beck, Wissen, ISBN 3-406-50866-9, S. 18 44. Rebecca et al., APPLICATIONS OF THE GV INSTRUMENTS TRACE GAS FOR METHANE AND ARBON DIOXIDE ISOTOPE STUDIES: LONDON DIURNALS AND IRISH WETLAND EMISSIONS Royal Holloway; University of London; http://www.cmdl.noaa.gov/ccgg/co2experts/presentations/fisher_r_69_p.pdf 99. Regnault & Reiset; Recherches chimiques sur la respiration des animaux. Ann. de chim. et de phys., 3ème série, 26 : 429. 114. Regnault, Annales de Chimie et de Physique, 1852, 3d ser, 36, S. 385 65. Regnault, V. & Reiset, J. (1849). Ann. Chim. (Phys.), 26 (3), 299. 66. Reiset ,J.A., Compt. Rend., T. 88, p.1007, T. 90, p.1144,1457 (1879-1880) 144. Reiset, J., Recherches sur la proportion de l´acide carbonique dans l´air ; Annales de Chimie (5), 26 1882, S. 144 33. Rogiers N. et al.; Labor für Atmosphärenchemie, Scherrer , Paul SEASONALVARIATION OFAND THE INFLUENCE OF LAND USE ON CARBON AND WATER VAPOUR FLUXES AT THE SWISS CARBOMONT FIELD SITE Institut, Villigen; Geographisches Institut, Universität Bern http://www.giub.unibe.ch/~eugster/publications/POSTER/Rogiers.2003.EGS.pdf 34. Rya, Steven, Quiescent Outgassing of Mauna Loa Volcano 1958-1994 Mauna Loa Observatory; Hilo, Hawaii 1995 http://www.mlo.noaa.gov/HISTORY/PUBLISH/steve/VolcCO2.htm 130. Ryan, Steven, Quiescent Outgassing of Mauna Loa Volcano 1958-1994, Mauna Loa Hilo, Hawaii Observatory; http://www.mlo.noaa.gov/HISTORY/PUBLISH/steve/VolcCO2.htm 81. Saussure de, T., Ann. chim. et phys., T. 2, 1816 [1830], p. 199. 50. Saussure de; Théodore, Sur la variation de l´acide de carbonique atmosphérique Annales de Chimie et Physique, 44[1830], S. 5 25. Schnell R. C. and the MLO Staff CMDL 1. OBSERVATORY, METEOROLOGY, AND DATA MANAGEMENT OPERATIONS 1.1. MAUNA LOA OBSERVATORY; http://permanent.access.gpo.gov/websites/www.cmdl.noaa.gov/publications/an nrpt23/chapter1_1.htm 97. Scholander P.F., J. MICRO GASOMETRIC ESTIMATION OF THE BLOOD GASES. IV. CARBON DIOXIDE; Biol. Chem. Scholander and Roughton 148 (3): 573. 96. Scholander, P. F.; ANALYZER FOR ACCURATE ESTIMATION OF RESPIRATORY GASES IN ONE-HALF CUBIC CENTIMETER SAMPLES ; J. Biol. Chem. 1947 167: 235-250 http://www.jbc.org/cgi/reprint/167/1/235 136: Scholander, Per F. (1905-1980 ), Biographical Memoirs V.56 (1987) National Academy of Sciences (NAS) 72. Schuftan, P, Chem. Fabrik, Nr. 51 1933 S. 513 93. Schuftan, P., "Gasanalyse in der Technik'. S. Hirzel Verlag,. Leipzig. (1931). 92. Schuftan, Paul and the early develeopment of gas- adsorption chromatography; Journal of high resolution chromatography, Vol. 8, Issue 10, S 651-658 1985 140. Schultze, F., Landw. Versuchsstationen, Bd. 9, 1867, S.217; Bd. 10, 1868, S. 515, Bd. 12, 1875 S. 1, Bd. 14, 1871, S. 366 5. Scotese, C.R., Bambach, R.K., Barton, C., Van der Voo, R., and Ziegler, A.M., Paleomap-Project; 1979. Paleozoic Base Maps, J. Geology, 87: 217-277. http://www.scotese.com/ 48. Segalstad, Tom V., The distribution of CO2 between atmosphere, hydrosphere, and lithosphere; minimal influence from anthropogenic CO2 on the global "Greenhouse Effect". Mineralogical-Geological Museum; University of Oslo http://www.uio.no/~tomvs/esef/esef0.htm 162. Selander, N.E., Bih. K.Sv. Vet. Akad. Handl., 1888 (aus Lundegardh (52) 155. Siegenthaler, U. 1984, 19th Century Measurements of atmospheric CO2, Comment Clim. Change 6, 409-411 6. Referenzen 119 128. Slocum,G. HAS THE AMOUNT OF CARBON DIOXIDE IN THE ATMOSPHERE CHANGED SIGNIFICANTLY SINCE THE BEGINNING OF THE TWENTIETH CENTURY, Monthly Weather Review: Vol. 83, No. 10, pp. 225-231. 83. Sonden, K. & Tigerstedt, R. (1895). Skand. Arch. Physiol. 6, I. 143. Stadterneuerung Dahme; http://www.dahme.de/html/altstadt/baugrund_3.pdf http://www.dahme.de/html/altstadt/sanierung.php 112. Stanhill, G., The Montsouris series of Carbon dioxide Concentration Measurements 1877 -1910, Climatic Change 4 (1982) 221-237) 118. Stepanova, Nina A. (1952). "A Selective Annotated Bibliography of Carbon Dioxide in the Atmosphere." Meteorological Abstracts 3: 137-170. 175 Steinhauser, F. Der Kohlendioxidgehalt der Luft in Wien und seine Abhängigkeit von verschiedenen Faktoren, Berichte des deutschen Wetterdienstes, Nr. 51, S 54, 1958 10. Stocker, A., T.F., H. Fischer, H.J. Smith, F. Joos, M. Wahlen, B. Deck, D. Mastroianni, J. Tschumi, T. Blunier, R. Meyer, B. Stauffer, High-Resolution Holocene CO2 -Record from the Taylor Dome ice core ( Antarctica), Indermühle, 1998, Nature, 398, 121-126 http://www.climate.unibe.ch/abstracts/indermuehle99nat.html 32. Studies on carbon flux and carbon dioxide concentrations in a forested region in suburban Baltimore ;John Hom et al. 2001; 1USDA Forest Service, Northeast Research Station, Indiana University, Bloomington,IN http://www.beslter.org/products/posters/johntower_2003.pdf 124. The national archives: http://www.archives.gov/research/guide-fed- records/groups/126.html 150. The Pre-1958 Atmospheric Concentration of Carbon Dioxide; EOS Meeting June 26, 1984 S. 415, 163. The Royal Society of Edinburgh, Fellowships http://www.rse.org.uk/fellowship/fells_indexp2.pdf 102. Thenard, Traité élém. de chimie, 5 edit., vol1, p.303 110. THOMAS, M. D. Precise automatic apparatus for continuous determination of carbon dioxide in air. Ind. & Eng. Chem., Anal. Ed. 5: 193-198. 1933. 125. U.S. STATIONS AND CAMPS IN ANTARCTICA http://quest.arc.nasa.gov/antarctica/background/NSF/facts/fact02.html 74. Uffelmann, Hygienische Topographie der Stadt Rostock, W. Werthers Verlag 1889,S. 45 73. Uffelmann, J. Luftuntersuchungen, Archiv f. Hygiene, 1888, S. 262 157. Uffelmann, J., Hygienische Topographie der Stadt Rostock, Wilhelm Werthers Verlag Rostock, 1889 156. Uffelmann, J., Luftuntersuchungen ausgeführt im hygienischen Institute der Universität Rostock, Archiv f. Hygiene, 8, 1888 S. 262 87. Van Slyke D. D. et al., Manometric analysis of Gas Mixtures, I,II Biol. Chem. 95 (2): S. 509 und 531. http://www.jbc.org/cgi/reprint/95/2/531 94. Vaupel, A., Mitarbeiter von W. Kreutz beim Deutschen Wetterdienst ; persönliche Mitteilung 2006 4. Veizer, J., Shaviv, Nir J.; Celestial driver of Phanerozoic climate? JOURNAL OF THE GEOLOGICAL ASSOCIATION OF CANADA; 2005 March Volume 32 No.1 http://www.gac.ca/JOURNALS/TOCs.html und http://www.fiz.huji.ac.il/~shaviv/Ice- ages/GSAToday.pdf 57. Warburg, Otto, Maßanalytische Bestimmung kleiner Kohlensäuremengen Zeitschrift f. Physiol. Chemie, Bd. 61, S. 261, 1909 89. West, John B., A Century of Pulmonary Gas Exchange; American Journal of Respiratory and Critical Care Medicine Vol 169. pp. 897-902, (2004) http://ajrccm.atsjournals.org/cgi/content/full/169/8/897 151. Wigley, T., The Preindustrial Carbon Dioxide Level; Climate Change 5, 1983 S. 315 -320 29. WMO Global Atmosphere Watch; World Data Center for Greenhouse Gases http://gaw.kishou.go.jp/wdcgg.html 161. Wollny, E., Forschungen aus dem Gebiete der Agrarkulturphysik, Bd. 8, 1885, S. 405 30. World Data Centre for Greenhouse Gases (WDCGG); http://gaw.kishou.go.jp/wdcgg/printedmatter/sum29/03co2.pdf 31. World Data Centre for Greenhouse Gases (WDCGG); WMO Greenhouse Gas Bulletin http://gaw.kishou.go.jp/wdcgg.html 6. Referenzen 24. 120 WORLD METEOROLOGICAL ORGANIZATION GLOBAL ATMOSPHERE WATCH No. 143; GLOBAL ATMOSPHERE WATCH MEASUREMENTS GUIDE; 2001/2003 http://www.wmo.ch/web/arep/reports/gaw143.pdf und http://www.wmo.ch/web/arep/reports/gaw148.pdf 154. WPC-53, WMO -Report of the Meeting in the CO2-Concentrations from pre- industrial Times I.G.Y 1983 41. Zhao, C.L., P.P. Tans, and K.W. Thoning, A high precision manometric system for absolute calibrations of CO2 in dry air, Journal of Geophysical Research, 102 (D 5), 5885, 1997. 26. Zustandsbericht 2001, Kapitel 3: The Carbon Cycle and Atmospheric Carbon Dioxide CO2 concentration trends and budgets, S. 3 http://www.grida.no/climate/ipcc_tar/wg1/pdf/TAR-03.PDF 6. Referenzen Danksagung: Für die freundliche Hilfe bei der Recherche historischer Literatur und Daten und Überlassung von Materialien bedanke ich bei Dr. L. Brake, Stadtarchiv Gießen Jana Farová, Infocentrum Město Tábor, (Cz) Ralph-Christian Mendelsohn, Deutscher Wetterdienst (DWD), Offenbach (D) Dr. Franziska Rogger, Archiv der Universität Bern (CH) Prof. Dr. Albrecht Vaupel, ehemals DWD (D) Dr. W . Wranik, Institut für Biowissenschaften, Meeresbiologie Rostock (D) New Sources: Journal of the American Chemical Society Vol. 36, No. 7: July 1914 GAS ANALYSES BY FRACTIONAL DISTILLATION AT LOW TEMPERATURES.2 G. A. Burrell and F. M. Seibert pp 1537 - 1548; 121