Notes on the Kinetic-Molecular Theory of Gases
advertisement

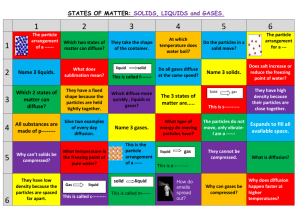
Notes on the Kinetic-Molecular Theory of Gases Name The kinetic-molecular theory of gases is based on the idea that An ideal gas is 5 Assumptions of Kinetic-Molecular Theory 1. 2. 3. 4. 5. Temperature Conversions between Kelvin and degrees Celsius K= C = Properties of a Gas ____________________ - a gas will completely fill any container it is in. ____________________ - gas particles, which are initially far apart can be crowded close together ____________________ - will be ~1/1000th the density of a solid or liquid phase ____________________ - gas particles glide easily past one another ____________________ - process of spreading out spontaneously to uniformly occupy a space Illustrate and describe Effusion Illustrate and describe Diffusion Rate of Diffusion – depends on two main properties of the gas particles; 1) Size or mass of molecules 2) Temperature of molecules Lighter and smaller gas particles will diffuse faster than heavier and larger particles. _________________ molecules will diffuse faster than _________________ molecules. Ideal Gas vs. Real Gas Ideal gas - completely conforms to the Kinetic-Molecular theory Real gas – Ideal Gases Real Gases List properties that are special to each!! 4 Variables used to describe gases 1) 3) 2) 4)