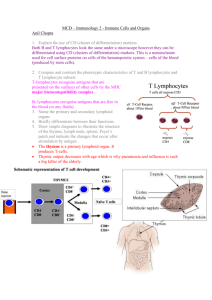
Disorders of the Immune System
advertisement

Immunology Immunity This is the ability to resist organisms or toxins that tend to damage tissues and organs. The immune system protects against exogenous (foreign) substances, microbial invasion, and possibly tumors. Occasionally, the immune response also damages normal host tissues and reacts to homologous antigens and sometimes endogenous antigens, the basis of autoimmune disorders. Native (Innate, Natural, Nonspecific) Immunity o General Information Aka “Low tech” Immunity. This is the immunity system with which we are born. This protects the patient against primary infections, i.e., pathogens that are encountered for the first time. Previous exposure is not required. o Types Anatomical barriers are created that seals area, protecting them against foreign invaders. Fatty acids of the skin inhibit growth of microorganisms. Cilia, normal bacterial flora, pH values, body temperature. Fluids that flush antigens away are also demonstrated in certain areas. Blood vessels are a great avenue to get macrophages to the site of infection to fight against the antigens. Macrophages and neutrophiles are activated by microorganisms and tumor cells. The antigens are then phagocytized and tumor cells may be lysed by natural killer cells. A complement cascade is also activated. Substrates are metabolized to enzymes, which can metabolize other substrates to enzymes. Complexes lyse invading organisms. Macrophages. o These are mononuclear, phagocytic cells that are critical in “processing” antigens for presentation to T lymphocytes. They produce a host of chemical mediators of immunity and inflammation (complement, chemotactic factors, cytokines, etc), and other things. Dendritic and Langerhan Cells o These function to “process” and present antigens to T cells in specific tissues. The dendritic cells are located in the lymphatic tissue, and the Langerhans’ cells are located in the epidermis. Natural Killer Cells o These attack “non-self” antigens without prior programming, i.e. nonspecifically. It is unclear how they actually do this, but they are considered important in certain viruses, bacteria, and cancer cell rejection. These are a first line of defense and account for about 10% of circulating lymphocytes. Major Histocompatability Complex o The Human Lymphocyte Antigen (HLA) complex is responsible for one’s own immune system to identify itself and do self no harm under normal conditions, but reject anybody else’s transplanted tissues. In spite of chemical suppression, etc, this has not been overcome for the perfect success of transplantation of most organs (corneas excepted due to the lack of vascularity), even in identical twins. o Class I proteins These are found on the surface of virtually all cells in a person’s body, except erythrocytes. o Class II proteins These are found only on the surface of macrophages and a few other cell types, such as B cells. Interferons, transferring, lactoferrin, complements, etc. o Example- The Eye Anatomical Barriers The eye is placed in a socket. Only the anterior end is exposed. It is also protected by the eyelids. Fluids Tears flush invading organisms from the surface. They also contain lysozymes, which is a natural antimicrobial. Vascularity High levels of blood vessels are located in the eye. Acquired (Specific) Immunity o General Information This is due to prior contact with the antigen to produce specific antibodies/ sensitized cells that can further fight infection at a later date. Native immunity efficiency is increased with acquired immunity. While the specificity of native immunity is general, protecting against all invaders, acquired immunity is very specific. For this reason, it is the basis of many laboratory tests. o Forms Cellular-mediated immune response This occurs inside of the cell. Antibodies (T cells) located on the cell’s surface have the antigens presented to them by APC (Antigen Presenting Cell). The activated T-helper cell secretes lymphokines, leading to the development of cytolytic T cells and a delayed-type hypersensitivity reaction. This form of immunity is lifelong. Once the antibodyantigen complex has formed, it is always present. A good example of this would be measles. Once a patient has had measles, it will never come back again. Humoral immune response This occurs outside of the cell. The antibodies (B cells) are located in the blood. The antigen binds to specific immunoglobulin receptors on the B cells, as well as being presented to the T-helper cell by the APC, which then causes it to secrete lymphokines. Lymphokines from T cells stimulate activated B cells to proliferate and to differentiate into antibody-producing plasma cells. Macrophages and T-helper cells are also activated. Unlike cellular immunity, humoral immunity is short-lived. It can be destroyed, filtered, etc. Boosters are required every several years to maintain immunity. Examples of this include tetanus and diphtheria Stimulation of Acquired Immunity Active o Actually getting the disease or a vaccination causes a mild case of the disease, but means that the antibodies are then found in the patient. Passive o Antibodies are already produced in another organism and then placed in the patient. An example of this would be antivenom for a snakebite. Components of Immunity Lymphatic System The lymphatic system is a specialized component of the circulatory system. Lymph fluid filters through the lymph nodes, which lie along the lymph channels. It kills microorganisms, via the production of lymphocytes and antibodies. It consists of a moving fluid (lymph) derived from blood and tissue fluid, and a group of vessels (lymphatics) and lymph nodes. From the lymphatic capillaries, lymph flows through progressively larger lymphatic vessels to eventually reenter the blood at the junction of the internal jugular and subclavian veins. Lymphatic movement through the body occurs by pressure gradients created through breathing and skeletal muscle contractions. There are two main components to the lymph circulation o Superficial system Drains skin Goes to three main groups of lymph nodes. These are the main groups of nodes that one palpates on routine physical exams. Cervical, axillary, and inguinal o Deep system Drains deeper structures The cervical axillary and inguinal nodes drain into the jugular, subclavian, and right and left lumbar lymphatic trunks respectively. Specific antigens for immunity are present in the lymphatic system of all vertebrates. Organization o This is a high pressure system which causes fluid to be constantly leaked out of the vessels. The fluid then fills the interstitial space. This is a problem since vital substances from the blood are lost such as electrolytes, glucose, etc. Also, with constant leakage, more fluid builds up in the interstitial space. If it is not returned to the vascular system, elephantitis and edema can occur, among other things. o Lymphatic vessels and capillaries return fluids to the capillaries. They are blind-ended, so all fluids flow out via muscle movements. o Everything drains to the venous system. Lymph capillaries vessels venous system 2/3 returns to the thoracic duct in the upper left thorax before traveling into the venous system. The remaining 1/3 goes to the right lymphatic duct. o Medium sized organized swellings are called lymph nodes. They have structure and are a port of the immune system. Lymph o Lymph is fluid derived from plasma that passes out of capillary walls into lymph vessels located in connective tissues around the blood vessels. o Lymph is cleansed of foreign particles at lymph nodes and is returned to the venous blood at particular sites, specifically at the right lymphatic duct and the thoracic duct. o Lymph drainage helps to prevent edema by removing proteins from the interstitial fluid. This decreases the oncotic force which draws fluid out of the capillaries. o Right lymphatic duct- drains the right, superior portion of the body. o Thoraic duct- drains the rest of the body. Formation and Function o The lymphatic vessels are a system of vessels that channel back into the circulation fluid and plasma proteins that have leaked into the interstitial space. The normal operation of the lymphatic system prevents edema. The lymph vessels empty into the subclavian vein. Lymph vessels have valves and rely on the pumping activity of surrounding skeletal muscle and smooth muscle in the walls of the lymph vessel. The lymphatics also transport fat absorbed from the gastrointestinal tract, to the circulating blood. Lymph nodes are periodic swellings of the lymphatic vessels where bacteria and other foreign material are removed from the lymph. Lymphoid Tissue o This is high pressure, so it leaks. o This collects fluid and protein from interstitial space and returns them to the blood. o It consists of reticular tissue (fibers and cells) and free cells (mostly macrophages. o May be diffuse (not sharply delineated and no special organization) or dense aggregations such as lymph nodules. o Nodules Aggregation of transitory, nonencapsulated lymphocytes in response to some stimulation (antigenic stimulation, etc). It usually consists of dense lymphoid tissue with a light central region, called the germinal center, and a darker, more peripheral cap or corona. Non-encapsulated dense spherical masses of lymphocytes within connective tissue in response to stimulation (i.e., antigen). Transient and may or may not be homogenous. Aggregates of lymph nodules are known as Peyer’s Patches. o Nodes Encapsulated structures along the lymph system with afferent and efferent vessels. The function of lymph nodes is to filter lymph to keep foreign materials such as bacteria out of the blood. Small kidney shaped organs that contain lymph nodules and fiber lymph to keep foreign material out of blood. The only lymphoid organ with both afferent and efferent vessels. Composed of Capsule- dense collagenous fibers encapsulating each node. Trabeculae- fibrous septa projecting inward from the capsule for support. It contains blood vessels. Reticulum- rectangular network of cells and fibers. o Thymus This is for the production of T-lymphocytes. The thymus is structurally composed of two distinct zones, the outer cortex and the inner medulla. The cortex is densely compacted with lymphocytes and surrounds a lightly staining central zone or medulla. A bi-lobed organ subdivided into thousands of lobules in the mediastenum. Each lobule consists of a clearly demarcated outer cortex and an inner medulla. Principle function is the production of T lymphocytes and modification of basic lymphocytes produced by bone marrow lymphopoiesis. This degenerates after birth. Lymphatic Drainage o Lymphatic vessels A network of channels that collect fluid from lymphatic capillaries. Structurally similar to veins, because they have thin walls. They may be differentiated from veins by their absence of RBCs, containing only lymph fluid. One-way valves allow lymph to flow in only one firection. Empties into large veins of neck via thoracic duct or right lymphatic duct. o Lateral drainage via Subauricular nodes Parotid nodes o Medial drainage via Submaxillary or submandibular nodes. Superficial Lymphatics of the head and neck o Lymphatic vessels: carry oxygen and nutrients from blood vessels to cells and carries wastes from cells back to the blood. Lymphatics of scalp Drainage Area Frontal Temporal and parietal Occipital Occipital and deep cervical nodes Lymphatics of External Ear Drains into the preauricular nodes, postauricular nodes, and superficial and deep cervical nodes. Lymphatics of face Drainage Area Eyelids and conjunctiva Cheek Side of nose, upper lip, and lateral lower lip Medial lower lip Temporal and infratemporal fossae Ending in… Preauricular and parotid nodes Postauricular and parotid nodes Ending in … Submandibular and parotid nodes Submandibular and parotid nodes Submandibular node Submental nodes Deep facial and deep cervical nodes Lymph Nodes of the Head and Neck o The lymphatic system of the head and neck converge about the jugular veins. The superficial lymph nodes of the head drain into the superior deep cervical nodes. The superficial lymph nodes of the neck have similar drainage. The deep lymph nodes of the neck all converge to drain into the supraclavicular nodes. o Lymph Nodes of the Head Lymph Node Occipital Posterior auricular Preauricular Parotid Facialinfraorbital Facial- buccinator Facialsupramandibular Location Near trapezius at the back of the head Behind the ear In front of the ear Embedded in or just below the parotid gland Below the orbit Angle of the mouth Over the mandible o Lymph Nodes of the Neck Lymph Node Submandibular Submental Superficial cervical Deep cervicalsuperior Location Under body of the mandible Between anterior bellies of digastric Along external jugular vein Along the carotid sheath, under the sternocleidomastoid muscle along the accessory nerve and internal jugular vein Deep cervicalinferior Along the carotid sheath, extending below border of sternocleidomastoid close to subclavian vein B Cells These are generated in the bone marrow from stem cells and undergo maturation and programming in the Bursa of Fabricius. Stem cells differentiate into cells of the erythroid, myeloid, or lymphoid series, with the last two groups migrating to the mammalian equivalent cells located in the tonsils, appendix, bone marrow, spleen, encapsulated lymphoid tissue, and lymph nodes (cortical follicules). Another guess is in the intestines. The maturation of B cells has two phases o The antigen-independent phase consisting of stem cells, pre-B cells, and B cells. o The antigen-dependent phase consists of the cells that arise subsequent to the interaction of antigen with the B cells. B cells constitute about 30% of the recirculating pool of small lymphocytes, and with a varying lifespan from about a few days to a few weeks. There are roughly 107 B lymphocytes. An antigen interacts with the B lymphocyte that shows the best “fit” with its immunoglobulin surface receptor (typically IgM). After the antigen binds, the B cell is stimulated to proliferate and form a clone of cells. These selected B cells soon become plasma cells and secrete antibody specific for the antigen. The plasma cells secrete thousands of antibody molecules per second for a few days and then die. Some activated B cells form memory cells which can remain quiescent for long periods but are capable of being activated rapidly upon reexposure to antigen. Most memory cells have surface IgG that serves as the antigen receptor, but some have IgM. The presence of these cells explains the rapid appearance of antibody in the secondary response. Function o These are made in response to antigens. The B cells divide and then transform to preplasma cells. These go to the medulla of the lymph nodes where they become plasma cells. At this point, they are taken through the venous system to the place of infection in the form of humoral antibodies at a rate of 2,000 per second. These antibodies are also called immunoglobulins. About 20% of the circulating lymphocytes are B lymphocytes. o There is also a memory function. When stimulated by an antigen, these “B” cells differentiate into plasma cells and secrete antibody to neutralize the specific antigen. o Most immunizations are B cell stimulators which are stimulated to create antibodies. T Cells There is an approximate ratio of 3:1 when comparing T cells to B cells. These are generated in the bone marrow, but then they travel to the thymus for maturation and functional development. The thymus is located in the mediastenum in the upper thorax and eventually degenerates after birth, but it is thought to produce a hormone to develop T cells. As T cell precursors pass through the thymus, cells reactive against “self” antigens are deleted, leading to immunologic tolerance to our own proteins. This is called “thymic education.” Competent (program) cells leave the thymus. 107 antibodies are possible. They migrate to the peripheral lymph nodes where they settle in the pericortical region inside of the cortex, as well as the spleen, tonsils, lymphoid tissue, and intestines. Other than going to a specific site, some T cells remain in the circulations to “patrol” the blood stream. The T cells account for 70% of circulating lymphocytes. All T cells have CD3 molecules on their surfaces in association with antigen receptors. CD3 molecules are involved with transmitting from the outside of the cell to the inside, the information that the antigen receptor is occupied. The T cells can be categorized depending on their function. o One type is involved with cellular immune reactions, where the cell itself “attacks” the antigen. There is a specific T cell clone (CD8) for each specific antigen. Some examples of involvement here are Transplant rejection Some viral and bacterial destruction Tumor rejection Everyone develops cancer, but most are rejected Memory This means that once one gets infected, one will not get sick again. o Regulatory functions of other immune processes as either T helper or T suppressor function. The T helper is devastated by the HIV family of viruses, so suppression takes over. These two categories balance each other. Four types based on biochemical markers. o T4/CD4 Cells These account for 60-80% of circulating WBC. In normal, healthy individuals, the CD4 to CD8 ratio is approximately 2:1. Types Helper o Gets the immune system going o Triggers maturation of T lymphocytes Inducer o Gets the immune system going o Affects T and B cells by getting them to react to antigens. Both play a role in delayed hypersensitivity. Secretions: Cytokines Lymphokines- secreted by T4 cells. o Interleukin-2- important in proliferation of T cells. Monokines- secreted by macrophages o Interleukin-1- activates T cells With advanced AIDS, T4 cells are drastically reduced CD4 count- count of T4 cells o Marks the disease progression o T8/CD8 Cells Types Killer/Cytotoxic o Kills antigens o Attaches to antigens and lyses cells, destroying them. Suppressor o Shuts down the immune system, suppressing the B cell immunoglobulin production and delayed hypersensitivity. Antigens Definitions o These are specific chemical substances in an organism or toxin that provoke a production of antibodies (an immune reaction) by the lymphocytes. They tend to be large molecules with high molecular weights (generally >10,000) in the structure of primarily proteins, but also polysaccharides or nucleic acids. Certain small molecules become immunogenic only when linked to a carrier protein. There is also required some form of chemical complexity. Typically they are foreign to the body under normal circumstances. One’s body has become tolerant, or learned to recognize their antigens so that the antigens are not attacked. When tolerance is lost, the antigens are attacked, resulting in what are known as autoimmune diseases. It is important to keep in mind that a specific antigen will illicit a very specific immune response normally. One antigen elicits one response. A hapten is an “incomplete” antigen that becomes complete when it combines with a body protein. Medications like penicillin are haptens. Surface radicals o These are small patches of about ten amino acids on the surface of foreign particles in specific configurations that do not change. They are used as markers and are the antigenic determinants. The amino acids are called epitopes The influenza virus constantly changes the epitopes, which is why patients cannot become resistant. The changing epitopes is the problem of vaccinations, which are only correct about 1/3 of the time. Viruses also have epitopes in pockets, which make binding impossible. Classification o Autoantigens These are a patient’s own antigens. They may cause autoimmune deficiencies. o Homologous Antigens These are antigens with minor differences in the proteins of the same species. These are what are used with ABO blood typing. o Organ Specific Antigens These are those antigens not exposed to the blood vascular system/immune system. For example, in the crystalline lens of the eye, cataracts are formed via liquefied proteins. During surgery, if the lens breaks open, proteins pours out, entering the blood vascular system. This can plug drainage, causing phacolytic glaucoma due to the antigens on the protein. o Cross reactivity This is when invading antigens are close to bodily antigens, causing antibodies to be produced killing the wrong antigens. Example: Antigens against strep Antibodies are formed that react with heart tissue, causing rheumatic fever. This scars the heart tissue and can cause murmurs. This was a larger problem before the advent of antibiotics. Antibodies/ Immunoglobulins Antibodies protect against infectious agents or their products by o Neutralizing toxins and viruses by two mechanisms Sterically interfering with the virus binding to its cellular receptor By crosslinking surface viral proteins, thus preventing uncoating of the virus. o Opsonizing microorganisms (making microorganisms more easily ingested by phagocytic cells). Antibodies are glycoproteins that are found in the plasma membranes of B cells in the blood and tissue fluids, composed. This means that each heavy chain has an N-linked oligosaccharide. Immunoglobulins are the basic units composing antibodies. As a group, they are known as gamma globulins and make up about 20% of the protein in blood plasma. The immunoglobulins are all tetramers, composed of four polypeptide chains hooked together by disulfide bonds. Two are short and light (MW of about 25,000), and two are long and heavy (MW of about 50-70,000). Light and heavy chains are subdivided into variable and constant regions. The constant region does not vary significantly between antibodies of the same subclass, and the variable regions are responsible for the antibody’s specificity and affinity to an antigen. These regions are composed of 3-dimensional folded. Repeating segments called domains. A light chain consists of one variable and one constant domain. Most heavy chains consist of one variable and three constant domains. IgM and IgE have four. The variable regions are responsible for antigen binding. The constant regions are responsible for various biological functions, e.g., complement activation and cell attachment. Each Y dimer has two identical Fab fragments which form the arms of the Y. The terminal end of the tetramer unit is custom made for attaching to a specific antigen (Fc region). The production of these immunoglobins takes place primarily in the lymph node follicles. There are five classes of immunoglobulins, corresponds to the differences in the heavy chains. o IgG IgG is the simplest form of an antibody, and it accounts for 75% of immunoglobins within the body (12mg/ml). There are four subclasses, IgG1 – IgG4. It is the major humoral antibody responsible for day-to-day antiviral, antibacterial, and antitoxin responses since it is long-lasting. About ½ is in circulation and ½ out in the tissues of the body, mainly the epithelial tissue (skin and conjunctiva). Function Fixation of complements (opsonization) Gamma Globulin is used to increase immunity o IgA o IgM It is the only class that can cross the placenta, therefore protecting the newborn in the first months of life. After 4 months, the newborn produces it own IgG. IgG production to a specific antigen begins 2-3 days after IgM first appears. This is the second highest percentage of total immunoglobulins found. It constitutes 15% of the circulating immunoglobulins (2mg/ml) and is the highest immunoglobulin level found in the human serum and that secreted on the surfaces of the body, i.e., in the saliva, sweat, tear film, GI tract secretions, etc, therefore it is known as the optometrist’s immunoglobulin since it is in the high concentrations in the tears. They are also secreted by the mammary glands, so they are the major antibodies in breast milk. It is the major antibody of colostrum. Half is found in the blood, and half is found in the tissue. There are two forms In the serum, it is seen as a monomer (single tetramer) In tissue, it is seen as a dimer (double tetramer) o When secreted, it is a dimer attached to a secretory piece which allows the passage of IgA to the mucosal surface and protects IgA from being degraded in the intestinal tract. Two dimers are joined by a lymphocyte Sometimes triple tetramers (trimers) are seen in the blood stream. It is secreted by epithelial cells (salivary glands, lacrimal glands, gut, and bronchus). Action Helps protect boundary tissue, especially in respiratory system by making the surface difficult for organisms to attach. The antigen has been used in many immunizations via delivery to epithelial/mucosal surfaces o The Sebin Polio vaccine is taken orally and stimulates IgA in the gut. o Salk’s vaccine is shot into muscle to stimulate IgG, because it is humoral immunity in extravascular space. This constitutes 10% of immunoglobulins (1mg/ml). It is mainly found circulating in the blood. It travels as a pentamer, five tetramers linked together, so it is the largest immunoglobulin with a high molecular weight. Although it has a fixing complement, because of the size, it is mostly in circulation and is usually the first immunoglobulin to be found in an acute infection. Its effects are short-term until other o IgD immunolglobulins can arrive. This has clinical diagnostic implication as is easily measured for a specific antigen. The function is protection of intravascular space (i.e., bloodstream). It can be produced by the fetus in certain infections. This constitutes 0.2% of immunoglobins (0.03mg/ml). It is found on the surface of lymphocytes as well as with IgM. It is quite sensitive to the action of heat and protein-digesting enzymes. The function is still being researched. There is a possibly autoimmune function. Perhaps, involved in the maturation of B lymphocytes. o IgE This constitutes 0.004% of immunoglobulins (0.0003mg/ml). It is found in increased concentrations in people with allergens. Function The normal role is to induce inflammation. o Blood cells exit the blood vessels and move towards the chemotactic source. The vessels dilate, allowing increased permeability. Fluid also leaks causing edema. It swells, and becomes red due to increased blood flow. Another function is anaphylactic allergic reactions. It forms antigen-antibody complexes which are important in initiating allergic responses (Type 1) when bound to the surface of mast cells and basophils. Protects against parasites. A polygenic role plays a factor. Response to Antigens o There are two responses, depending on the antigen Primarily viral and tumor transplant rejection produce cellular immunity (T-Cells), Bacterial and viral reinfection produce humoral immunity produced (B-Cells). o Whether a T cell or B cell is activated is thought to be genetic. Consequences of Antigen-Antibiotic Reactions o An antibody is specific for a particular antigen. o Antibodies bind to the epitopes of antigens. This is a noncovalent interaction that is weak in nature. The strength of its binding is proportionate to the fit of the antigen with its antibody combining site. This is the key of the immune system. The next are courses that the reaction may follow: Precipitation No cells are involved. This could either be bivalent (two binding sites) or multivalent (multiple binding sites). Everything combines to form a 3-dimensional lattice, allowing phagocytic cells to ingest antigens. Agglutination This links many antibodies to antigens, and involves cells. It is the basis for many clinical tests, such as blood typing, pregnancy tests, etc. It relies on acquired immune and specific antigens. Activation of Complements A complement is an enzyme found in the blood, which is normally inactive. This is bound and not found free, because it could cause damage. The complement system consists of approximately twenty heat labile proteins that are synthesized in the liver and released into the circulatory system. Several of these are proenzymes, which must be cleaved to form active enzymes. Three main functions o Lysis bacteria, allografts, and tumor cells. A pore is produced in the membrane called the membrane attack complex which allows water and electrolytes into the cell. o It attracts phagocytic leukocytes as well as mast cells which release mediators involved in inflammation. o Opsonization Complements are activated by the antigen-antibody reaction. The antibody recognizes the antigen. When attached, a site is revealed that acts as the complement. The complement attaches (fixes) to the antigen via antibodies. This is done via a nine step cascade. The product (Membrane Attack Complex- MAC) eats its way through the cell wall via lysis. The result is that fluid flows into the cells and bursts. Two pathways that lead to the production of C3b, the central molecule of the complement cascade Classical pathway o This is initiated only by the antigen-antibody complexes. This pathway will lead to cell lysis, therefore it is a reaction to the membrane-bound antigens. This occurs rapidly and efficiently o This includes a biological cascade or amplification, since a single antigen molecule can induce the formation of many effector molecules (C3b). o Here, the Fc region of an antigen-antibody complex binds the C1q subunit of C1. This binding reaction activates esterase activity of C1 and results in the cleavage of C2 and C4 which forms C2b4b, which is called the C3 convertase. o C3 convertase can cleave many C3 molecules to C3b and C3a. C3a is released and C3b forms a trimolecular complex with C2b4b (C5 convertase). This interacts with the C5 to break off C5a. C5b combines with C6 and C7 into a C5b67 complex. Further binding of C5b67 with C8 and C9 produces C5b6789, the membrane attack complex (MAC) which causes lysis by imbedding itself in the microbial plasma membrane, creating leaky channels in the membrane. Water and salt then enter into the cell and disrupt the intracellular ionic environment and kill the microbe. Alternate pathway o This has a slower activation. C3 is activated directly by many unrelated cell surface substances such as bacterial lipopolysaccharides (endotoxin), fungal cell walls, and viral envelopes, also activate the complement cascade, bypassing C1 and C2. This leads to more inflammatory effects. o This pathway is more important in the first time the body is infected by a microorganism since an antibody is not required to activate this pathway as it is in the classical pathway. Phagocytosis This is the process of engulfment where the cell attaches and ingests particles to destroy them. o Adherence is called opsonization, where the antigens are coated with antibodies. o Phagocytosis is due to two different cells. Microphages Origin Neutrophiles Lifespan Short (nondividing) Motility Very mobile. They are like little “suicide bags”. The granules are packets of enzymes that digest cells. Defense Against pyogenic bacteria (pus formers, i.e., staph and strep) Macrophages Monocytes Long (nondividing) Attaches to organs and remains there. They act to screen the blood, attacking and ingesting foreign bodies by creating a mononuclear phagocytic system. Attaches to the liver, lymph nodes, spleen, bone marrow, and lungs. Combats bacteria, viruses, and protozoa living in the cells. Both exhibit Diapedesis- can leave the blood stream Ameboid motion- very slow (40 microns/min) Chemotaxis- moves toward chemicals o Influenced by inflamed tissues Cytokines Messenger Molecules of the Immune System Inducing and regulating the immune responses by stimulating various immune cells to grow, differentiate, or synthesize specific products is very complicated and involves multiple interactions between lymphocytes, monocytes, PMNs, and endothelial cells. Chemical protein messengers are prominently involved and are generally classified as “cytokines.” Cytokines induce their effects in three ways o Autocrine effect- They act on the same cell that produces them. o Paracrine effect- They affect other cells in their vicinity. o Endocrine effect- They affect many cells systematically. Cytokines mediate their effects by binding to specific high affinity receptors on their target cells. Production of cytokines and responsiveness to cytokines are tightly regulated by environmental stimuli and other cytokines. Cytokines have incredibly short half lives. Functions include activating T cells, communicating with CD8 cells, stimulating B cell differentiation, regulating chemotaxis and lymphocyte growth, as well as activating inflammatory responses. Mediators affecting lymphocytes o IL-1 produces mainly by macrophages to activate a variety of target cells, e.g., T and B lymphocytes, neutrophils, epithelial cells, and fibroblasts, to grow differentiate and produce IL-2. IL-1 is called the endogenous pyrogen, which acts on the hypothalamus to cause the fever associated with infections/inflammations. o IL-2 is produced mainly by helper T cells (also B cells) and stimulates both helper and cytotoxic T cells to grow. o IL-4 and IL-5 are produced by helper T cells promoting the growth and differentiation of B cells. IL-4 may also enhance IgE synthesis. Mediators affecting macrophages and monocytes o Chemotactic factor attracts monocytes to inflamed areas via diapedesis, at which point they become macrophages. o Migration inhibitory factors may act to retain macrophages at the site of a delayed hypersensitivity reaction. o Macrophage-activating factors produced by lymphocytes activate macrophages to phagocytize certain organisms. Mediators affecting polymorphonuclear leukocytes o Leukocyte-inhibitory factor inhibits migration of neutrophils analogous to migration-inhibitory factor. o Chemotactic factors for neutrophils, basophils, and eosinophils selectively attract each cell type. Mediators affecting stem cells may stimulate growth and differentiation of bone marrow cells as well as the production of granulocytes and macrophages. Mediators with other effects o Interferons are three low molecular weight glycoproteins (IFN-α, IFN-β, and IFN-γ) IFN-α and IFN-β are strongly induced by viral infection and dsRNA. Both bind to surface receptors on neighboring cells and induce the synthesis of enzymes that render these cells more resistant to infection. IFN-γ is a lymphokine synthesized by activated T cells and is one of the most potent activators of phagocytic activity in macrophages, NK cells, and neutrophils. It also enhances antigenpresentation and increases antibody production in B cells. o Tumor Necrosis Factor is released from monocytes or macrophages and has many effects. It increases lymphokine and IL-2 synthesis by T helper cells. It increases B cell proliferation. It attracts and activates neutrophils. It is induced by lipopolysaccharides and is an important mediator of endotoxin-induced septic shock. It reduces the utilization of fatty acids in adipose tissue. Besides its tumorcidal properties, it is an important mediator in the inflammatory response and can activate phagocytosis by macrophages. There are also lymphokines produced by the lymphocytes and monokines produced by the monocytes that are important in the production of messengers. Immune Mechanisms of Tissue Injury o Somehow our own body defense system can run amok and do damage to our own tissues. These are somehow polygenic, and the antigen can be either exogenous (from outside the body) or endogenous (from within, that is reacting to our own tissues). Interferons Interferons are a heterogenous group of glycoproteins produced by human and other animal cells after viral infection. They are a part of the innate immunity and inhibit the growth of viruses by blocking the translation of viral proteins. There are mainly three types of interferons. Alpha, beta, and gamma interferons are named responsively based on their cell of origin: leukocytes, fibroblasts, and lymphocytes. o In a viral infection, gamma interferon is secreted by helper T cells and natural killer (NK) cells, and it stimulates cytotoxic T cells, NK cells, and macrophages to be more potent killer cells. Besides helper T cells and NK cells, several different cell types secrete other interferons into the extracellular fluid during infection. o Alpha and beta interferons are secreted by virally infected cells and these interferons act on homologous neighboring cells to induce anti-viral activity. As an example, an infected liver cell will secrete alpha or beta interferon and the interferon will bind to plasma membrane receptors on nearby liver cells, whether they are infected or not. The strong inducers of these interferons are viruses and double-stranded RNAs. The extensive list of inducers makes it clear that induction of these interferons is not specific. Their inhibitory action is not specific for any virus. They are typically specific in regard to the host species in which they act: i.e., interferons produced by human cells are active in human cells but are much less effective in cells of other species. Interferons inhibit the intracellular replication of a wide variety of viruses. They act by binding to a cell surface receptor which induces three proteins that prevent the translation of viral mRNA without affecting the translation of cellular mRNA. Because interferons are produced within a few hours of the initiation of viral replication, they may act in the early phase of viral disease to limit the spread of virus. In contrast, antibody begins to appear in the blood several days after infection. By binding to the plasma membrane, interferon triggers the synthesis of several enzymes in the cell. If the cell is infected or eventually becomes infected, these enzymes block the synthesis of viral proteins, which inhibits viral replication. Some research has shown that interferon is effective in treating HIV. However, keep in mind that interferon is not specific and cannot recognize different viruses. Nonspecific Mediators Complement o This is a set of 9 proteins circulating in the blood in an inactive form. Once activated, they rapidly decay. o Complement C3 is the “king-pin” that activates the rest of the cascade. o Enzymes activated by bacterial endotoxins activate C3 (alternative pathway) or antibody molecules combine with the antigen flex and can activate C1, which splits and reacts with C2 and C4. C2 and C4 combined can serve as an enzyme to activate C3 (classical pathway). o The complment (source of complement: MΦ) cascade, an important nonspecific humoral component, can be triggered directly by an invading organism or by antibodies formed in the course of a specific immune response. Complement, in turn, plays an important role in modulating the function of neutrophils and macrophages. Macrophages are not only important effector cells in the expression of innate immunity, but also, as antigen-presenting cells, they play an important role in triggering specific immune responses mediated by B and T lymphocytes. The latter mediate cellular immunity and in addition they regulate the function of antibody-producing B cells, as well as that of macrophages and natural killer cells. Cytokines o Protein hormones secreted by immunologically active cells in response to external stimuli. These molecules mediate the immune response. Lymphokines o These are molecules secreted by lymphocytes, i.e., IFNg (interferon gamma). Increases the effectiveness of the macrophages and natural killer cells. B cells are also stimulated by interferon. The induction and regulation of immune response involve multiple interactions among cells of the immune system, many of which result from the release of polypeptide mediators. Depending on the source of the mediators, they have been called lymphokines (lymphocyte-dervied) or monokines (monocyte-derived). However, some mediators originally thought ot be produced only by lymphocytes or monocytes can be generated by many non-lymphoid cells, and the term cytokines is now preferred. Cytokines mediate their effects by binding to specific high-affinity receptors on their target cells. Cytokines induce their effects in three ways o They act on the same cell that produces them o They affect other cells in their vicinity o They affect many cells systemically. Production of cytokines and responsiveness to cytokines are tightly regulated by environmental stimuli and other cytokines. Immunologic Physiology Tumor Immunology Animals carrying a chemically or virally induced malignant tumor can develop resistance to that tumor and cause its regression. In the course of neoplastic transformation, new antigens called tumor-associated antigens (TAA), develop at the cell surface and the host recognizes such cells as “nonself.” Mechanism o Cell-mediated reactions attack these “nonself” tumor cells and limit their proliferation. Such immune response probably acts as a surveillance system to detect and eliminate newly arising clones of neoplastic cells. In general, the immune response against tumor cells is weak and can be overcome experimentally by a large dose of tumor cells. Some tumor cells escape surveillance by modulation, or internalizing the surface antigens so that affected tumor cells in vitro no longer provide a target for immune attack. o The cell-mediated response includes natural killer cells, which act without antibody. Killer cells, which mediate antibody-dependent cytolysis. Cytotoxic T cells. Activated macrophages. o Tumor antigens can stimulate the development of specific antibodies as well. Some of these antibodies are cytotoxic, but others enhance tumor growth by blocking recognition of tumor antigens by the host. o Spontaneously arising human tumors may have new cell surface antigens against which the host develops both cytotoxic antibodies and cellmediated immune response. o Lymphocytes activated by interleukin-2 may be useful in cancer immunotherapy. Cancer cells do not obey feedback controls that normally stop growth and reproduction. May be due to irritant, radiation, virus, or genetics. Tumors may or may not stimulate an immune response. o If stimulated, T-lymphocytes directly kill infected cells and recognized tumor cells. Helper T cells and interferon activate natural killer cells which non-specifically attack tumor cells and viral infected cells. Oncogenes o An oncogene is any gene that encodes a protein capable of transforming cells in culture or inducing cancer in animals. Their discovery has had a major impact on research involving the fundamental mechanisms of carcinogenesis. o The term oncogene itself is derived from the Greek word, “oncos,” which means tumor. o Oncogenes are mutated forms of normal cell genes called protooncogenes. o Proto-oncogenes can become activated to express oncogenic potential in several ways. Point mutation Choromosomal rearrangement Gene amplification Viral insertion o Proto-oncogenes are important for the control of normal cell growth and division. When mutated they can become carcinogenic oncogenes resulting in Excessive cell growth due to overproduction of growth factors Excessive cell multiplication due to flooding of cell with replication signals. Transplantation Immunology An autograph (transfer of an individual’s own tissue) is always accepted. A syngeneic graft is a transfer of tissue between genetically identical individuals (e.g., identical twins) and usually “takes” permanently. A xenograph is a transfer of tissue between different species and is always rejected by an immunocompetent recipient. An allograft is a graft between genetically different members of the same species, e.g., from one human to another. Unless specific measures are taken, it is rejected by the “allograft rejection.” o A T cell mediated reaction is the main cause of rejection of many kinds of grafts, eg, skin, but antibodies contribute to the rejection of certain transplants, especially bone marrow. o The acceptance and rejection of a transplant is determined, in large part, by the class I and II MHC (major histocompatability complexglycoproteins found on cell surfaces) proteins, with class II playing the major role. Class I and certain class II antigens are detected by using a panel of known antibodies plus complement to lyse donor lymphocytes. In addition to the tests used for matching, preformed cytotoxic antibodies in the recipient’s serum reactive against the graft are detected by observing the lysis of donor lymphocytes by the recipient’s serum plus complement. This is called “crossmatching” and is done to prevent hyperacute rejections from occurring. Among siblings in a single family, there is a 25% chance for both haplotypes to be shared and a 50% chance for one haplotype to be shared. For example, if the father is a haplotype AB, the mother is CD, and the recipient child is AC, there is a 25% chance for a sibling to be AC, i.e., a 2-haplotype match, and a 50% chance for a sibling to be either BC or AD, i.e., a one-haplotype match. Well-matched transplants of bone marrow may establish themselves initially in 85% of recipients, but subsequently, a graft-verses-host (GVH) reaction develops in about 2/3 of them. This reaction occurs because grafted immunocompetent T cells proliferate in the irradiated, immunocompromised host and “reject” cells with class II antigens, resulting in severe organ dysfunction, especially in the skin, liver, and intestinal tract. To reduce the chance of rejection of transplanted tissue, immunosuppressive measures, eg, cyclosporine A, corticosteroids, azathioprine, and radiation are used. Cyclosporine A prevents the activation of T lymphocytes by inhibiting signal transduction within the cytoplasm of T cells. This drug is well-tolerated and is remarkably successful in preventing the rejection of transplants. Immunosuppressive drugs also reduce the GVH reactions. Tissue Transplantation and Graft Rejection The portion of the genome determining immunologic identity is called the Major Histocompatibility Complex. In man it is the HLA (Human Leukocyte Antigen). This is a gene cluster on chromosome #6. Part is inherited from the mother, and part from the father. Careful HLA typing is essential for successful organ transplant. HLA mismatch could cause graft rejection. HLA types must match between donor and recipient. Rejection is type II hypersentivity reaction. This is a very complex process in which both cell-mediated immunity (T cell-mediated reactions) and circulating antibodies (antibody-mediated reactions) play a role. Group of about 25 different antigens in cell membranes. Only 4 exist in any one person. Try to match donors combination with recipients. Prevent rejection with anti-lymphocyte serum (decreases rejection reaction) and glucocorticoid therapy (cortisone), ACTH, etc. These drugs leave the body vulberable to disease. Suppresses formation of antibodies and lymphocytes. X-ray and chemotherapy suppresses rejection. Immunological Tolerance Tolerance is a specific immunologic unresponsiveness, i.e., an immune response to a certain antigen (or epitope) does not occur, although the immune system is otherwise functioning normally. In general, antigens that are present during embryonic life are considered “self” and do not stimulate an immunogenic response. The lack of an immune response is the result of the deletion of selfreactive T cell precursors in the thymus. On the other hand, antigens that are not present during the process of maturation are considered “nonself” and usually elicit an immunologic response. The main process by which T lymphocytes acquire the ability to distinguish self from nonself occurs in the fetal thymus. This process, called clonal deletion, involves the removal of T cells that react against antigens present in the fetus at that time. Even exogenous substances injected into the fetus early in development are treated as self. Whether an antigen will induce tolerance rather than an immunologic response is largely determined by the following o The immunologic maturity of the host, e.g., neonatal animals is immunologically immature and will accept allografts that would be rejected by mature animals. o The structure and dose of the antigen, e.g., a very small molecule induces tolerance more readily than a complex one, and very high or very low doses of antigen may result in tolerance instead of an immune response. Purified polysaccharides or amino acid copolymers injected in very large doses result in “immune paralysis” or a lack of response. Other aspects of the induction or maintenance of tolerance are as follows o T cells become tolerant more readily an remain tolerant longer than B cells. o Administration of a cross-reacting antigen tends to terminate tolerance. o Administration of immunosuppressive drugs enhances tolerance, eg, in transplantation. o Tolerance is maintained best if the antigen continues to be present. Disorders of the Immune System This is an explosive area of research and is truly a hotbed, as it is clinically important to all health professionals. In optometry, contact lenses, medications, solutions, the various diseases and disorders themselves all involve both abnormal and normal immune responses. There are three major areas of concern o Autoimmune Diseases o Immunodeficiency o Misdirected immunity, aka hyperimmunity or hypersensitivity Monoclonal Disorders of the Immune System These are immune deficiencies due to genetic abnormalities or secondary to drugs or infections. It is a disease entity in which there is a disproportionate proliferation of a single cloe of immunoglobulin-forming cells. Plasma cell disorders o Monoclonal neoplasms related by B-lymphocyte lineage (e.g., Multiple myeloma and Waldenstrom’s macroglobulinemia) o Benign Monoclonal Gammopathy Here the monoclonal immunoglobulin is found in the serum by eletrophoresis, but other wise all is normal. Some of these individuals may go on to develop multiple myeloma. o Multiple Myeloma This involves a spread of neoplastic cells throughout the bone marrow. o Waldenstrom’s macroglobulinemia Here there is also a proliferation of abnormal lymphoid cells that secrete IgM. Burton’s X-linked agammaglobulinemia due to leukemia or lymphoma o Males have few Ig bearing B-lymphocytes Antibody deficiency o Decreased production of IgA by B cells, most frequent immunodeficiency. Common varied immunodeficiency o Low levels of IgG and IgA due to failure to differentiate to plasma cels. DiGeorge’s Syndrome o Congenital T cell deficiency. All lymphocytes are B cells. Small thymus. Cardiac defects. Autoimmunity Disorders There are many diseases that fall under this category, but there are four general mechanisms which have been postulated to account for loss of self-tolerance. More than one defect might be present in each disease, and defects vary from one disorder to the other. o Bypass/Escape of T helper cell tolerance Unresponsiveness to a “self” antigen may be maintained by tolerance at the T cell level. Such tolerance may be terminated by cross-reactions, i.e., when the host responds to antigens that crossreact with tolerated “self” antigens. For example, in rheumatic fever, antibodies against streptococcal antigens cross-react with heart tissue antigens. o Imbalance of T suppressor-helper function In normal immune regulation, suppressor T cells may limit an immune response to “self” antigens. If suppressor T cell function decrease antibodies to “self” antigens, eg, an antibody to normal, IgG, may be formed. Antibodies such as IgM and IgG occur in rheumatoid arthritis, in which antigen-antibody complexes form in joints. o Emergence of a sequestered antigen Certain tissues such as sperm, CNS, and the lens and uveal tract of the eye, are sequestered so that their antigens are not exposed to the immune system. When such antigens enter the circulation accidentally, such as with damage, they elicit both humoral and cellular responses, producing aspermatogenesis, encephalitis, or endophthalmitis, respectively. o Idiotypic bypass mechanisms Furthermore, the pathogenesis of autoimmunity appears to involve immunologic, genetic, and viral factors interacting through complicated mechanisms that are poorly understood. Either humoral or cell-mediated reactions can be involved, but most are antibody-mediated. Many autoimmune diseases exhibit a marked familial incidence, which suggests a genetic predisposition to these disorders. In recent years, a growing number of diseases have been attributed to autoimmunity, but in many the evidence is not firm. This is because autoantibodies can be found in the serum or tissues of a remarkably large number of apparently normal individuals, particularly in older age groups. Systemic Lupus Erythematosis Rheumatoid Arthritis o This is a Type 3 disease which affects more females than males, 3:1. The target tissue is usually synovial joints but can hit the eye, skin, kidney, heart, lungs, and other tissues. The complexes activate complement, leading to severe inflammation in the involved tissues. It is polygenic with an unknown trigger. Adults with RA do not have the associated iritis that occurs in JRA. Spondyloarthropathies o Here the target tissue tends to be the spine and sacroiliac joints. Affected people, polygenic, usually are HLA-B27 positive, and the trigger is often an infection with Chlamydia, or enteric bacteria like shigella or salmonella. There is commonly an associated immune mediated (not infectious) urethritis, conjunctivitis, and uveitis along with arthritis. It is slightly more common in males than females. Reiter’s syndrome is an example. Sjorgen’s Syndrome o Characterized by dry eyes (keratoconjunctivitis sicca) and dry mouth (xerostomia), it is due to an immune mediated destruction of the lacrimal and salivary glands. This is most commonly seen in association with an autoimmune disease, usually RA in about 50-60% of cases. Therefore, it is usually a Type 3 disease. Systemic Sclerosis o This is also called scleroderma and is characterized by inflammation and fibrosis of the skin, kidneys, intestines, and other organs of the body. It is somehow autoimmune, but poorly understood. Polymyositis o This is also called dermatomyositis. Here the target tissue is skin and muscle with severe chronic inflammation of unknown cause. About 40% of patients get a peculiar “heliotrope” discoloration around the eyes. This is one of the few autoimmune diseases which are dominated by males. Necrotizing Scleritis o This is associated with autoimmune diseases and has a 30% mortality within 5 years. Amyloid o This is a heterogenous group of pathogenic fibrillar proteins that accumulate within tissues and organs, either because of excess synthesis or because of resistance to catabolism. New observations provide strong evidence that in most patients, some derangement in the immune apparatus underlies this disease. Scleroderma Immunodeficiency This is a circumstance in which the patient cannot fight off certain diseases, especially infections. It can occur in any of the fours major components of the immune system: B cells, T cells, complement, or phagocytes. Primary Immunodeficiency o These are primarily inherited problems in T or B cells, and there are a host of specific diseases. These are almost always genetically determined. They are typically seen between 6 months and 2 years of age as maternal antibody protection is lost, and are noted due to the susceptibility of infants to recurrent infection. These are “bubble babies” which must always be protected. o B Cell Deficiencies X-linked hypogammaglobulinemia (Burton’s gammaglobulinemia) Very low levels of immunoglobulins and a virtual absence of B cells. Maybe be due to failure of maturation of pre-B cells. Clinically recurrent pyrogenic infections occur in infants about 6 months of age when maternal antibody is no longer protective. Select immunoglobulin deficiencies IgA deficiency is the most common of these. o T Cell Deficiencies Thymic Aplasia (Digeorge’s Syndrome) Causes a profound deficit of T cells. Chronic Mucocutaneous Candiasis The skin and mucous membranes of children are infected with Candida albicans, which in immunocompetent individuals is a nonpathogenic member of the normal flora. These children have a T cell deficiency specifically for this organism. Other T cell and B cell functions are normal. o Combined B and T cell deficiencies Severe Combined Immunodeficiency Disease (SCID) Recurrent infections caused by bacteria, viruses, fungi, and protozoa occur early in life, because both B cells and T cells are absent. This inherited disease is probably due to a defect n the differentiation of an early stem cell. Adenosine Deaminase and Nucleoside Phosphorylase Deficiency Affects bone marrow differentiation Wiskott-Aldrich Syndrome Ataxia-Telangectasia o Complement Deficiencies Patients with C2 and C4 deficiencies have diseases resembling SLE or other autoimmune diseases. Secondary Immunodeficiency o These are acquired as a result of some condition, infection, disease, etc. which is developed. Examples include: malnutrition (most common), advanced cancer, advanced age, severe disease of a vital metabolic organ, advanced or uncontrolled diabetes, and some infections like AIDS. o B Cell Deficiencies Common Variable Hypogammaglobulinemia Patients present with recurrent infections caused by pyrogenic bacteria. The number of B cells is normal, but the ability to synthesize IgG (and other immunoglobulins) is greatly reduced (idiopathic). T cell function is usually normal. o T Cell Deficiencies AIDS Patients with AIDS present with opportunistic infections caused by certain bacteria, viruses, fungi, and protozoa. This is due to greatly reduced helper T cell numbers caused by infection with the retrovirus human immunodeficiency virus, HIV. This virus specifically infects cells with the CD4 surface receptors. Markedly reduced helper-tosuppressor T cell ratios occur, but elevated immunoglobulin levels are found. The response to specific immunization is poor. Measles T cell function is altered but immunoglobulins are normal. AIDS The HIV virus causes a drastic reduction of the T4 lymphocytes (helper cell line), leading to severe acquired immunodeficiency. It is a collapse due to only one defect. Resulting problems include cancer (lymphoma, Kaposi’s sarcoma, etc.), opportunistic infections (CMV, pneumocystis carinii pneumonia, Candida and Cryptococcus neoformans meningitis, mycobacterium tuberculosis, etc), and ultimately death. The virus must enter the bloodstream and then CNS cells, macrophages, T lymphocytes, and other cells. It is primarily spread by sexual secretions and blood or blood products. Introduction o A variety of ophthalmic disorders have been attributed to the acquired immune deficiency syndrome (AIDS). They generally fall into four groups. Vascular disorders Infections Neoplasms Neuro-ophthalmic disorders o These disorders are either caused directly by the human immunodeficiency virus (HIV) or caused indirectly by the diminished immunocompetent state of the infected individual. o Due to significant changes in the understanding of the pathogenesis of HIV infection, the ability to measure blood levels of HIV activity, and the antiretroviral drugs to control HIV replication, many patients with AIDS have experienced striking changes in their health status, especially fewer opportunistic infections. History o Pathogenesis o The HIV Virus HIV is a retrovirus. It can be found in the blood stream, destroying T4 cells in the process. Normal T4 count is 800-1200 cells per cubic ml. When the T4 cell count is decreased, opportunistic organisms can cause infection. o Viral replication The HIV attaches to the CD4 locus on the T helper cell. Its core of RNA penetrates the T cell wall. The RNA is transcribed into the DNA by HIV’s enzyme: reverse transcriptase, and is integrated into the host cell’s DNA as “proviral DNA.” Proviral DNA acts as a template for production of both messenger RNA (for later protein synthesis) and genomic RNA molecules. The new virus (virion) is assembled from genomic RNA and protein chain. After assembly and during budding and release from the T cell surface, the long protein chains are “snipped” by HIV’s enzyme protease, which causes the new virions to be infectious. o Viral dynamics After initial infection with HIV, viral production stabilizes at a “set point” within months. This represents a relatively stable level of production of HIV and is measured as “viral load” or the number of copies of RNA per mL of plasma in the peripheral blood supply. Since the number ranges from hundreds to millions, viral loads are measured in log units (i.e. 2.0 log = 100 copies, vs. 5.0 log = 100,000 copies, etc). In general, 100 billion are produced per day, but not all are infectious. Quantitative polymerase chain reaction (PCR) tests measure the RNA in the plasma, with a sensitivity limit of 200 copies/ml. Newer versions will be as sensitive as 20 or 50 copies. The branched DNA (bDNA) has a sensitivity limit of 400 copies. Either measurement is valid. The new “set point” and resulting viral load reflecting chronic HIV replication are now felt to govern the spread of destruction of CD4 cells, progression to clinical AIDS, and risk of death. Viral load is also indicative of the success of combination antiretroviral therapy, with greater reductions in viral load correlating to reduced risk of disease progression and efficacy of the drug regimen. o The Disease Process Window Period Once a person is infected, it takes 2-8 days before the virus replicates. Viral replication can be accompanied by flu-like symptoms, chills, fever, malaise, etc. Detectable Antibodies Approximately 2 weeks after infection, antibodies will be detectable in the blood stream. Up to 3 months after infection, 96% of those infected will test positive for antibodies. By 1 year after infection, 99% will test positive for antibodies. Up to 1% may never show positive antibody testing. HIV Screening OraQuick Oral Fluid Rapid HIV Test o Specimen is gathered from gums and mouth by individual or fingerstick by technician. o The specimen is tested immediately in a solution. Results are seen in 20 minutes. o Sensitivity: 99.3%, Specificity: 99.9% OraQuick 3-Step Test o A fingerstick whole blood sample is collected and the specimen collection loop is inserted into the tube of blood. o The blood-filled end of the loop is inserted into the Developer Solution Vial and stirred to mix. o The flat pad is inserted into the vial containing the blood sample. Results are available in 20 minutes. Reveal HIV-1 Antibody Test o www.reveal-hiv.com/maintest.htm Uni-Gold Recombigen HIV Test Positive Testing The standard tests used for HIV tests for HIV antibodies are the ELISA and Western Blot. It is possible to test blood for presence of the virus, but it is not commonly used for screening. Modes of Transmission o Body Fluids Blood, semen, vaginal fluids, and breast milk have all been implicated in HIV transmission. HIV has also been found in tears and saliva, but not in sufficient concentrations to result in infection. o Transfusion There is an estimated chance of 1 in 40,000 of getting the virus through transfusion. o Needle Stick There is an estimated chance of less than 1% of getting the virus through an accidental needle stick in a health care setting. Postexposure prophylaxis (PEP) is recommended to decrease seroconversion. A protocol has been developed by the CDC to help with decision making. o IV Drug Use Shared needles containing infected blood which many cause infection. This has a very high risk, especially in certain communities, but there are no published estimates overall. o Sexual Intercourse All heterosexual and homosexual activities put the participants at risk. The highest risk practices are visiting prostitutes and practicing anal sex. o Health Care Providers Health care providers face a greater risk of occupational blood exposure or needle stick injuries. Through Dec 2000, there have been a total of 56 documented cases and 136 possible cases of occupational transmission. The highest risk group is nurses. Optometry is not considered a high risk profession. External Ocular Disease (Vascular Disorders)- This is seen in 90% of patients. o Conjunctival microvasculopathy This is a disorder of conjunctival vasculature, seen next to the limbus. The most apparent area seems to in the inferior perilimbal area of the bulbar conjunctivae. It is found in almost every AIDS patients, particularly if CD4 count is low, meaning that it is often used as an indicator of treatment success. Changes include Capillary dilation Isolated vascular fragments Vessel segments of irregular caliber and corkscrewing Microaneurysms due to lumen breaking down Granular appearance to blood column within blood vessels due to the increased aggregation of red blood cells Decreased flow rate, with “sludging” The changes are similar to those seen in individuals with sickle cell disease or diabetic retinopathy. Its etiology remains unknown, possibly due to immune complexes and/or endothelial cell involvement. It is seen in those with elevated fibrinogen levels, which are possibly a major contributing factor to this rheologic disorder o Treatment None for conjunctival vascular changes. They are self-resolving. Consult an infectious disease specialist, and be sure that they receive the proper spectrum of care. External disease (specifically infectious diseases) o Herpes zoster ophthalmicus (HZO): 5-15% HZO developing in any “young” individual should raise the suspicion of concomitant HIV infection, since HZO usually does not occur in individuals under the age of 50. These patients experience worse signs than the elderly, but less pain. A study done in NY found that 61% of individuals under 44 years of age were members of groups at high risk for AIDS. Older patients are considered to be at lowest risk for HIV infection. HZO may be the first manifestation of AIDS (relatively small percentage of cases), but 30% or more of AIDS patients may have episode(s) of herpes zoster. There are the typical signs and symptoms of HZO in those with AIDS. Keratitis and anterior uveitis are possible as parts of HZO (but 50% of AIDS patients had little or no ocular disease when treated early with oral acyclovir) HZO sometimes is less frequent in patients with CD4 count between 100-300 and is more common in patients with CD4 < 100 or > 300-400 Treatment Aggressive therapy with oral acyclovir at 800 mg 5x/day for 7-10 days, started within 2 days of onset of rash Alternative drugs include famciclovir 500 mg tid or valcyclovir 1g tid. IV acyclovir is used in patients with advanced immunodeficiency or with disseminated zoster. o o o o o Oral steroids should be avoided unless there is progressive proptosis with ophthalmoplegia or optic neuritis induced by varicella zoster virus. Mild analgesics (acetaminophen) should be tried first, but typically must use controlled substances (Tylenol 3 with codeine, Vicodin, etc.) for the pain. Herpes Simplex Keratitis (HSV) This occurs in HIV-infected individuals, but not to a greater degree than in seronegative individuals, but there is a greater recurrence rate in HIV-positive patients (2.5x higher). AIDS were thought to have a predilection for ulcerative keratitis in the corneal periphery and limbal area compared to HIV-negative individuals, but there are essentially no differences in presentation. Diagnosis is the same as that of those without HIV. Treatment Topical Viroptic, standard doses (q2h) for 9-12 days provided normal healing and resolution. The time to resolve is not significantly different from HIV-negative patients Fungal Keratitis Immunocompetent patients usually show fungal involvement only after ocular trauma, steroid therapy, ocular surface disease, or corneal insult. HIV + individuals can spontaneously develop fungal infections Bacterial Keratitis: <5% Bacterial infections may occur in patients with AIDS, though this is not a common feature of the syndrome. HIV status does not predispose patients to bacterial corneal ulcers, but HIV+ individuals who develop corneal ulcers are difficult to treat due to decreased immune defense mechanisms. Risk factors include contact lenses, as well as dry eyes (which is found in 20% more HIV+ patients than the age-matched control patients. Early detection and aggressive topical therapy is critical in HIV+ patients. It is wise to use a combination antimicrobial therapy for 21+ days. K. Sicca: 10-20% This is the most common ocular manifestation since HIV attacks the lacrimal gland. Tears are needed as a primary barrier, especially with contact lens wearers. These can lead to corneal ulcers. Treat with artificial tears tid-qid. Conjunctivitis Cytomegalovirus (CMV) CMV has been identifies in the conjunctiva of selected AIDS patients through vascular transmission, although the conjunctiva is rarely affected. Shedding of CMV in tears has also been reported. Transmission of CMV through contact with tears of AIDS patients has never been reported in literature. Transient Conjunctivitis This has been noted in about 10% of AIDS patients. It responds well to topical antimicrobials, as would be prescribed for typical cases in HIV negative patients. Recurrence rates are variable. Other types of conjunctivitis HIV + patients are subject to non-specific conjunctivitis, which are culture-negative, as well as chronically red eyes from keratitis sicca. o Molluscum contagiosum: 5% These are lesions on the skin caused by an infection with the pox virus. Unlike molluscum found in kids, those in AIDS patients may be typical or as granulomatous lesions, often larger (10mm or larger) and in increasing numbers (as many as 100). This is not common in AIDS patients. Most cases are reported in patients with severe immunodeficiency (CD4 count <100). Treatment These may be excised surgically, but they often recur. Many physicians do nothing since they could possibly resolve after HAART. Experimental cryotherapy for periocular lesions, using intraocular cryotherapy unit instead of dermatologic liquid nitrogen. External disease (neoplasia) o Kaposi’s sarcoma (KS) This is a frequent manifestation of AIDS, presenting in anywhere from 24% (1980s) to 15% (today) of AIDS patients. 85-905 of those with KS occurred in male patients with AIDS. Intrathoracic involvement was seen in 33%. The etiology is controversial. A newly discovered herpes virus is implicated in virtually all cases of KS (human herpes virus type 8). Presentation The reddish-brown macular lesions are most obvious on the skin (face, legs, trunk), but are more severe and lifethreatening when visceral (lungs, GI tract). They increase in number and coalesce. Geographic (worldwide) and internal (multi-organ involvement) suggest a viral role. Sites of tumors are vascular channels of endothelium of lymph and/or blood vessels. The palpebral conjunctiva and eyelid are typical ocular sites. About 20% of patients with cutaneous KS will have ophthalmic KS as well. The eyelids and conjunctiva are involved due to their high vascularity. They can also be seen on the roof of the mouth if found on the eye. o Eyelid KS appears equally on upper and lower eyelids. They may mimic an unresponsive hordeolum. o Conjunctival KS occurs more frequently in the inferior fornix. It may be mistaken for chronic subconjunctival hemorrhages, a foreign body granuloma, or a cavernous hemangioma. o Eyelid or conjunctival KS may be the first clinical manifestation of AIDS. About 4% of patients show ophthalmic signs as the first manifestation of AIDS. o Most patients are asymptomatic with ocular KS o Symptomatic KS will cause cosmetic disfigurement (#1 reason for therapy), as well as trichiasis (most serious indication for therapy), and local irritation or recurrent subconjunctival hemorrhage. Treatment of KS The most important clinical manifestation requiring treatment is trichiasis, although cosmesis is a more frequent reason for therapy, due to the stigmatizing nature of KS. Types o Chemotherapy (local therapy) o Radiation therapy (best for conjunctival KS) o Cryotherapy o Local surgical excision (very difficult due to high recurrence rates) o Systemic therapy for AIDS with HAART (reports of KS stabilizing or regression after initiation of antiretroviral therapy). Prognosis Chemotherapy or radiation result in total regression in some cases, but recurrences are frequent within 6-12 months. Ocular structures hold up well to radiation therapy It is also cost-effective therapy. Orbital Manifestations o Orbital Cellulitis o Non-Hodgkin’s Lymphoma (NHL) Diffuse NHL increases frequent with reduced immunocompetence (CD4<100). NHL most often involves the central nervous system, GI tract, liver, and bone marrow. Orbital involvement is very infrequent (only 3% of all cases reported). Diagnosis Proptosis, pain, eyelid edema, and purple discoloration (ecchymosis) are all common signs. Systemic symptoms of NHL include drenching night sweats, fever, and weight loss (known as “B symptoms”). Treatment High likelihood of systemic involvement A combination of chemotherapy and radiation in lower doses has been increasingly successful in prolonging survival. o Burkitt’s Lymphoma (???) Neuroophthalmic Manifestations o Papilledema o Cranial nerve palsies o Ocular motility disorders o Visual field defects Iridocyclitis in AIDS patients o With CMV retinitis, the anterior chamber reaction is very mild. It is difficult to appreciate an anterior reaction with mild cells, no PAS, and few or no KPs. o A toxoplasmic retinitis would result in a likely iritis, varying in severity with potentially severe flare/cells, KPs, and a red/painful eye. o Tuberculosis typically is chronic, insidious, with mutton fat KPs; resistant to local therapy. It may respond to systemic therapy as pulmonary or extrapulmonary TB is treated. Rifabutin can induce a uveitis. o Syphilis is classically severe, non-granulomatous, with heavy fibrin and a possible hypopyon. It is also common to have a neuroretinitis (optic nerve involvement with swelling, papillitis, or retrobulbar optic neuritis, as well as patchy/ plaquoid retinitis, vasculitis/phlebitis, and vitritis). o Prophylaxis for Mycobacterium avium complex disease with rifabutin results in an infrequent report of hypopyon uveitis, suggesting a devastating endophthalmitis, without an infectious cause identified. This is typically seen in patients taking rifabutin with either clarithryomycin or fluconazole, which both inhibit liver cytochrome enzyme P450A3 responsible for metabolizing rifabutin. o Scattered reports of low grade, chronic anterior chamber reactions without obvious cause are also seen. o Diagnosis Dilate all AIDS patients regardless of anterior chamber reaction, due to the high likelihood of a retinitis. The differential diagnosis of toxoplasmosis, syphilis, tuberculosis, and CMB depends on the CD4 count, clinical features, serology, and possible blood culture. Referral for systemic evaluation. o Treatment This depends on the diagnosis of the underlying systemic disease. For rifabutin-associated uveitis, topical steroids and cycloplegics are very effective, with change in drug therapy for MAC. Varicella Zoster Retinopathy o Although the definitive mechanism is unknown at this time, the virus probably reached the retina by neural pathways from the brain. o This is the most devastating eye infection in AIDS, since it is both rapidly progressive and without consistently effective treatments. The condition is distinctive enough to be initially named “progressive outer retinal necrosis” (PORN), and later renamed “rapidly progressive herpetic retinal necrosis” (RPHRN). o Symptoms and signs Painless, rapid fading of peripheral or central vision History of previous cutaneous herpes zoster (most common) or other varicella infection (possibly months to years earlier) Deep, homogenous retinal opacification with initially discrete area, then rapidly confluent (occurring within several days to 2 weeks) Multifocal or confluent areas of retinal opacification in either posterior pole and/or retinal periphery Very high risk of RD (up to 80%) of cases due to extremely thinned retina. A detachment is much more common than in CMVR. Vasculitis is not too common (about 25% of cases) and occurs later in the disease (contrasted with acute retinal necrosis or “ARN” which has profound vasculitis, plus vitritis/iritis superimposed on the retinal destruction, occurring mainly in immunocompetent patients) RPHRN has no granular borders and few or no hemorrhages (compared to CMVR) RPHRN can destroy the entire retina in two weeks. o Treatment Aggressive antiviral therapy with high dose acyclovir (IV) plus either foscarnet or ganciclovir (also IV) The greatest successes have used two IV antiviral drugs in combination with additional local therapy (IV injections) in an attempt to quiet the retinitis. o Prognosis Generally a dismal prognosis. Many patients declined to NLP. There is better success for stabilizing retinitis with combination therapy. The impact of HAART at this time is uncertain. Toxoplasmic Retinitis o This is the third most common infectious cause of retinitis after CMVR and RPHRN. It appears to occur during the course of a newly acquired primary toxoplasmosis infection or from dissemination of the toxoplasmosis microbes from other sites of infection. Rarely, if ever, does it occur adjacent to a previous, quiet retinal toxoplasmosis scar, as in immunocompetent patients. o The CD4 count is typically <100 for toxoplasmosis in AIDS patients. More than 50% of AIDS patients who has toxoplasmic retinitis have associated encephalitis (which is life-threatening) o Symptoms and signs Floaters and reduced vision Moderate to severe vitritis Frequent anterior chamber reaction, often severe Retinitis is very thick, dense, and opaque. It is more opacified than with CMVR, and also without the dry/granular borders characteristic of CMVR. Hemorrhages are infrequent in toxoplasmic retinitis in AIDS. o Treatment Oral pyrimethamine (Daraprim), 25-100mg/day Sulfadiazine (4-8g/day in 4 divided doses) plus leucovorin (525mg/day) to reduce bone marrow suppression Clindamycin (Cleocin) 300 mg qid is the alternative drug for patients with sulfonamide allergy Oral corticosteroids are deferred as long as possible, then used in minimum doses o Prognosis Regression occurs in 7-10 days, then inactivation occurs in about 5-8 weeks (with 75-90% of patients responding). Relapses are frequent. Toxoplasmic encephalitis must be managed for life with suppressive therapy. The impact of HAART in treating this is uncertain at this time. o Other drugs with anti-toxoplasmosis effects Atovaquone is mainly used for the acute treatment of pneumocystis pneumonia of moderate severity Azithromycin and clarithromycin have some success, but have inconsistent results against toxoplasmosis when used singly. Dapsone is used mainly as a prophylaxis for pneumocystis pneumonia, but has dual prophylactic efficacy against toxoplasmosis. Syphilitic Retinitis o Patients with AIDS have a fairly high incidence of associated syphilis. Ocular manifestations of syphilis are often more severe, prolonged, and harder to treat in AIDS patients. o Retinitis occurs during the secondary stage of syphilis. Retinitis is strongly associated with neurosyphilis (meningitis or meningovascular syphilis with cerebrovascular accidents). There is no correlation between a depressed CD4 count and the severity of the disease. o Signs and symptoms Very common to have optic nerve involvement (papillitis, retrobulbar neuritis, peri-neuritis). Phlebitis and vasculitis are also very common. The retinitis can appear dry and grainy, similar to indolent/granular CMVR, but can also present as necrotizing, yellow-white, deep lesions, which are plaquoid. Syphilitic eyes are markedly inflamed, while the eyes with CMVR are rarely inflamed at all. A severe fibrinous, non-granulomatous iritis is associated with syphilis, also a variably severe vitritis. o Treatment Lumbar puncture, with evaluation for neurosyphilis Treatment for retinal syphilis is typically the same as for neurosyphilis Aqueous penicillin, 2-4 million units, delivered IV every 4 hours for a minimum of 10 days (up to 14 days). The total amount of medicine is between 12-24 million units of penicillin. For penicillin-allergic patients, use doxycycline 100mg, bid, po, for 21 days. Acute Retinal Necrosis Syndrome Bacterial/ Fungal Retinitis Retinal Microvasculopathy AIDS/HIV retinopathy o This should not be confused with CMV retinitis. o Cotton Wool Spots (CWS) are the most frequently encountered ocular complication of AIDS. They occur in <10% of patients with ARC or AIDS-related complex disease (weight loss, oral candidiasis, oral hairy leukoplakia(white patch on the tongue that does not scrape off since it is not epithelial), herpes zoster, but CD4>200) It occurs more often as CD4 count drops. About 50% of patients with CD4 <50 have HIV retinopathy. CWS usually present near large vessels in the posterior pole. The proposed mechanism is a rheologic abnormality (increased fibrinogen and viscosity, with focal retinal vessel infarction). This is a poor prognostic sign for future ocular disease. With a very low CD4 count, CMV retinitis is more likely, as well as an increased potential for hematologic spread of other retinal pathogens. o Diagnosis Rule out DM, SLE, and HTN. Both DM and HTN have additional retinal vascular changes. Other possibilities include vasculitis, SLE, anemias, malaria, and other diseases. Differential from CMV retinitis Follow-up in 6-8 weeks. CWS will fade or appear elsewhere, but CMV lesions will smolder, enlarge, and advance. o Treatment There is no specific treatment for CWS. They rarely affect the visual acuity and disappear in about two months. A few reports have associated CWS with Pneumoscystic carinii pneumonia (PCP). The doctor should inquire about dyspnea or a nagging, non-productive, chronic cough. Refer for a chest X-ray, sputum analysis, broncho-alveolar lavage, etc. CMV retinitis (CMVR) o Aka Pizza Pie Retinopathy/ Ketchup and Cottage Cheese Retinopathy o This is the most common cause of infectious retinitis in AIDS patients. The range of frequency of CMV retinitis ranges from 5-40%, with an average of 25% of all AIDS patients, but these frequencies are prior to the use of HAART. Frequencies of new cases of CMV infection have dropped as much as 83% in the past 2-3 years. o Survival after the diagnosis of CMVR has also increased from 6 weeks (pre-1987) to 1+ years with improved prophylaxis and treatment of opportunistic infections (mid-1990s). With HAART, patients are generally given 18-24 months to live and 12 months without. Blindness or the threat of dying without sight was the primary reason for suicide in AIDS patients o About 1% of cases of CMVR occur as the presenting disease (AIDSdefining opportunistic infection) in undiagnosed AIDS patients, however most patients manifest CMVR after a diagnosis of AIDS and occurrence of other opportunistic infections (most often PCP). CMVR is most common when the CD4 count is <50. Average CD4 count per many studies was 25-30 at the time of CMVR diagnosis. o Routes of CMV transmission to the eye With decreasing immune suppression of CMV, it reactivates and spreads hematogenously to either the anterior or posterior retinal circulation, then into the contiguous retina. Alternatively, it may spread via the optic nerve into the eye Regardless, it spreads into the adjacent retina and results in fullthickness destruction of the retina (necrosis). o Symptoms of CMVR Gradual onset of floaters, which are frequently overlooked by patients. Photopsia Scotomas (most likely with posterior pole involvement) No pain, redness, or photophobia Diagnosis rests on clinical appearance with DFE. There are no definitive serologic tests. o Clinical features of CMVR Two classic patterns Hemorrhagic or fulminant (typically posterior pole) o White, necrotic retina o Retina is grainy early on, then later becomes opaque o Multiple hemorrhages o Spreads along vascular arcades o May mimic BRVO, if hemorrhages are very extensive Indolent or granular (typically retinal periphery) o Fewer hemorrhages o Grainy leading edge o Small satellite or foci at or beyond (ahead of) the leading edge o Most often seen in the periphery “Brushfire” nomenclature refers to the leading edge of retinitis which is yellow/white and grainy, with atrophic retina left behind with ghost retinal vessels, mild RPE loss, and gliosis. CMV involvement of the optic nerve head appears as a yellow/white disc with small hemorrhages surrounding the margins. Peripapillary retina may also be involved Hard to delineate from other disc edema Risk of retinal detachment is about 30-50% after 1 year of therapy This is due to multiple small holes in the periphery which thins the retina. There is greater risk of RD with larger amounts of retina involved, particularly in the periphery. Treated with vitrectomy and silicone oil, but post-op VA is typically poor (about 20/100) and silicone oil causes a marked hyperopic shift, which is permanent. o Treatment of CMV Four virustatic agents presently used Decreases CMVR within 1-2 weeks, but the medications must be taken chronically. Ganciclovir (dihydroxypropoxymethyl guanine of DHPG) o After phosphorylation by a CMV-associated enzyme, it inhibits DNA polymerase and viral proliferation. o Routes of administration include: IV, intravitreal injection, oral capsules, or Vitrasert, a sustainedrelease device. o Intravenous treatment- Doses are 5mg/kg, used twice daily during induction (2-3 weeks) and once daily during maintenance until the retinitis relapses due to poor trough levels of drug obtainable with IV therapy (average is about 60 days to reactivation), then re-induce for 2-3 weeks followed by maintenance. o IV injection of ganciclovir has 13 hours half-life. Risks include endophthalmitis (uncommon), RD (uncommon), and development of extra-ocular CMV infections due to lack of systemic effect of ganciclovir. o Sustained-release intraocular implant device (Vitrasert) releases the drug over 6-8 months without systemic toxicity or systemic anti-CMV protection. This greatly improves the quality of life, without a Hickman catheter, IV regimens, or catheter-related risk of sepsis. The cost is $4000 per device, along with a surgeon’s fee of about $1500. o The standard of care may be Vitrasert plus oral ganciclovir prophylaxis to prevent fellow-eye CMVR or extra-ocular infection (colitis, esophagitis, pneumonia). The frequency of felloweye retinitis was reduced from 38 to 22% with Vitrasert plus oral ganciclovir. o Oral ganciclovir is used for maintenance therapy after standard IV induction therapy or prophylaxis and cases of peripheral retinitis only, since oral bioavailability is only 6%, and the times to retinitis reactivation are shorter. It is about 50% effective as a prophylactic drug. o Side effects include neutropenia, granulocytopenia (about 25% of patients), and significant thrombocytopenia (<5%). Granulocytopenia can be managed with granulocyte colony stimulating factor (Epogen). Foscarnet (trisodium phosphonoformate): IV or intravitreal injection o This does not require phosphorylation, but it acts similarly to ganciclovir. o It is used almost exclusively as an IV therapy (induction followed by maintenance) at 90mg/kg twice daily during induction, followed by 90mg/kg daily during maintenance, until relapse occurs. o Foscarnet has been used intravitreally, alone, or in combination with ganciclovir, in cases of visionthreatening retinitis. It has also been used intravenously in a dual therapy regimen with IV ganciclovir for sight-threatening retinitis. o Foscarnet is about 2.5x more costly than ganciclovir and is additionally more difficult to use, requiring pre-hydration with saline as well as a controlled pumping device. o An early study done by the SOCA (Studies of Ocular Complications of AIDS) found that patients started on foscarnet IV lived about 4 months longer than patients started on ganciclovir IV (8 mo vs 12 mo), possibly due to the anti-HIV effects of foscarnet (study dates from 1992). o Side effects include nephrotoxicity (33% of patients) and electrolyte abnormalities. Side effects can be decreased if saline is used first. o Do not use this with Cidofovir = kidney damage Cidofovir (HPMPC) o This is not phosphorylated as is ganciclovir. It is potentially active against CMV resistant to ganciclovir and/or foscarnet. o Routes of administration include IV or intravitreal injection. o Cidofovir has an exceptionally long half-life, therefore it is used much less frequently than ganciclovir or foscarnet. o IV treatment is 5mg/kg. Induction is once weekly for 2 weeks. Maintenance is once biweekly. o Single intravitreal injections have controlled retinitis for up to 55 days, but toxicities (hypotony, iritis) are worrisome and intravitreal use is not FDA approved. o This is very toxic to the kidneys. Concomitant probenecid must be used (which causes rash, nausea, and fever in 50% of patients) or saline. o Iritis is quite common (up to 25% of patients), somewhat more frequent with longer use of cidofovir. o Do not use this with Foscarnet = kidney damage Fomivirsen (ISIS 2922) o This is an anti-sense oligonucleotide drug which mimics DNA, binds to RNA, and interferes with protein synthesis. o The only route of administration is intravitreal injection and IV. o Fomivirsen is used for patients intolerant of other treatments or who were insufficiently responsive to other treatments. o It is long-acting, typically used once weekly for 3 weeks during induction, then once monthly. o Toxicities include intraocular inflammation (vitritis, uveitis) and RPE stippling. New Developments Valganciclovir (valine ester of ganciclovir) is an oral prodrug. The oral bioavailability is about 60%, and the AUC is equal to that of IV ganciclovir. It is being tested for both treatment and prophylaxis at this time. Serologic tests to identify patients most at risk for CMV end organ disease are being studied. They are measuring either CMV pp65 antigen or CMV DNA levels. Antiviral Therapy o Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Drugs competitively bind to HIV’s reverse transcriptase enzyme and also mimic the nucleoside components needed to make DNA. These drugs are incorporated into the expanding DNA chain produced through reverse transcription, which results in “false DNA” as well as an inactive or non-infectious virus. Examples AZT (azidothymidine), zidovudine; Retrovir o This resembles thymidine. It is phosphorylated to AZT-triphosphate, which is recognized by the reverse transcriptase, but once used by the enzyme, the next nucleotide cannot be added to the growing chain. ddI (dideoxyinosine), didanosine; Videx o This acts in a similar way. ddC, zalcitabine; Hivid d4T, stavudine; Zerit 3TC, lamivudine, Epivir 1592U89, abacavir, Ziagen 300mg AZT + 150mg lamivudine; Combivir o Non-nucleoside reverse transcriptase inhibitors Drugs only bind directly to the reverse transcriptase enzyme and distort its shape, without being able to mimic the nucleoside components of DNA. The end result is still production of a defective or non-infectious virus. Examples Nevirapine; Viramune Delavirdine; Rescriptor Efavirenz; Sustiva o Protease Inhibitors Drugs inhibit the scissoring action of HIV's protease enzyme, resulting in the production of a non-infectious virus. Examples Saquinavir hard gel; Invirase Saquinavir soft gel; Fortovase Ritonavir; Norvir Indinavir; Crixivan Nelfinavir; Viracept Amprenavir; Agenerase o Fusion Inhibitors This is mainly for end stage AIDS patients. It stops the virus from infecting new CD4 cells. Side effects include rashes at the injection site. Examples T-20 (Virturide) Fuzcon Recommended Antiretroviral Drug Regimens o The standard of care currently uses one protease inhibitor (not saquinavir hard gel) plus two different nucleoside reverse transcriptase inhibitors. Choice of saquinivir soft gel (Fortovir), ritonavir, indinavir, or nelfinavir; amprenavir. Plus one of the following pairs (which are compatible without overlapping drug toxicities) AZT plus 3TC (one pill is Combivir) d4T plus 3TC d4T plus ddI AZT plus ddI AZT plus ddC o Alternative regimens now becoming more popular Efavirenz plus one of the nucleoside reverse transcriptase inhibitor pairs above (called a “protease-sparing regimen”) Two protease inhibitors (most often ritonavir with saquinavir hard gel) with one or two nucleoside reverse transcriptase inhibitors (dual protease inhibitors are most useful when the pre-treatment viral load is very high). o Less frequently used combinations Nevirapine plus one of the nucleoside reverse transcriptase inhibitor pairs above. Delaviridine plus one of the nucleoside reverse transcriptase inhibitor pairs above. o Generally it is not recommended to use any pair of nucleoside reverse transcriptase inhibitors, because they are not able to provide sufficient suppression of viral replication. It is definitely not recommended to use monotherapy with any drug, whether nucleoside reverse transcriptase inhibitor, non-nucleoside RT inhibitor, or protease inhibitor. Impact of HAART on AIDS care o HAART is Highly Aggressive AntiRetroviral Therapy. It is a specific regimen tailored to each individual patient. o The purpose is a complete “reconstruction” of the immune system. Changes occur in immune cellular function with an increase in the number of “memory cells” (CD4 cells that retain memory of specific pathogens or microbes), as well as “naïve cells” (which may later be exposed to pathogens and recognize them). This is seen as an increase in the patient’s CD4 count. o There are many case reports of patients with intractable or difficult opportunistic infections which improve significantly after initiating HAART. Examples are diarrhea from cryptosporidiosis, Kaposi’s sarcoma, and progressive multifocal leukoencephalopathy. o HAART also caused a significant drop in the number of hospital days, hospital admissions for AIDS-related illnesses, hospice days, skilled nursing days (home care nursing), and referrals. Though pharmacy expenses have increased staggeringly for HAART, these increases are offset by large savings in healthcare delivery (particularly hospitalizations). o Many report a significant reduction in new cases of CMV retinitis, as high as 83% less cases of end-organ CMV disease. The retinitis generally stabilizes and some do not required further maintenance treatment. The criteria for stopping CMVR maintenance include a stable CD4 count >100 and a viral load reduction of 2.0 logs for at least 6 months plus 6 months or more of healed or inactive retinitis. o This can cause Immune Recovery Retinitis/ Uveitis. Some instances of patients who have started HAART and were not previously diagnosed with retinitis, but develop retinitis, viritis, or papillitis within 8-12 weeks of initiating HAART have been noted. This is only seen in patients with a previous attack of uveitis/ retinitis, generally those with HAART and a very low CD4 count. The drugs actually cause the uveitis. The infectious antigen in the anterior chamber is attacked by HAART. Then the immune system inflames before the attack, causing the IRR. The consensus is that it requires three months of unsuccessful HAART, with both viral load reductions and increases in CD4 counts, before the patient has some “immune reconstruction” and is protected against CMVR. These patients must be dilated every week. Recommendations for optometric practice in relation to HIV-related individuals o Protection to the patient There is no evidence that HIV has been transmitted through any diagnostic procedure performed in optometric care. Because HIV has been detected in tears and the ocular surface epithelium, the following should be noted: Handwashing is the #1 most effective means to avoid the risk of transmitting or acquiring infection in the course of examination. If an open wound is present on the examiner’s hands, disposable gloves must be worn. Gowns and masks are not needed for normal optometric procedures. o Spaulding Classification Body Contact Intact Skin Mucous Membranes Sterile Body Cavity Disinfection Required Low level High level Sterilization FDA Device Class Non-Critical Semi-Critical Critical Critical Care High risk of infection if contaminated Including o Surgical instruments o Needles o Implants o Cannulae Purchased sterile/sterility maintained Sterilization by o Autoclaving o Ethylene oxide o Chemical sterilants/ disinfectants 2% gluteraldehyde 6% hydrogen peroxide Peracetic acid Peracetic acid and hydrogen peroxide Orthopharaldehyde (OPA) Semi-Critical Care Contact mucous membranes or nonintact skin Including o Thermometers o Tonometer tips o Gonioscopy lenses o PMMA contact lenses Must be free from all microorganisms Require o Wet pasteurization o High level disinfection with chemical disinfectants (glutaraldehyde room temperature for 20 minutes, hydrogen peroxide) Bleach 1:10 bleach solution for 15 min Noncritical Care Contact intact skin, but not mucous membranes Including o Blood pressure cuffs o Phoropter o Slit lamp rests Cleaning is all that is required between patients (70% IPA) o Procedures with potential for exposure or requiring sterile techniques Injections, FANG, tissue cultures and biopsies, infected patients, lacrimal probe and irrigation, foreign body removal, electrocautery, and laser due to the aerosol effect. o Instrument disinfection Patient contact instruments Routine cleaning of phoropter, slit lamp, keratometer, etc, with alcohol is impractical and unnecessary because HIV is a fragile virus. Goldmann tonometer Simple cleaning of the tonometer tips with 70% isopropyl alcohol provides adequate protection against HIV 1:10 dilution of household bleach is also highly effective. 3% hydrogen peroxide is effective, but the solution needs to be changed twice daily 70% isopropyl alcohol soaking is effective, but there is danger in glazing the plastic tonometer tip. Diagnostic CL Soft CL o Disinfected with peroxide or heat system RGP o Disinfect with peroxide or chlorhexidine-containing system o The outer and inner surfaces of the lens and case should be wiped with alcohol. For added protection, the inner cup may be filled with 1:10 bleach solution, then washed out and air dried immediately. Hand-held instruments (spud, forceps, tweezers) 1:10 bleach solution Peroxide Autoclaved, then sealed and packaged (gold standard) Best advice Establish sanitary and disinfection procedures on a routine basis for all patients, and there will be no need to be alarmed about HIV patients. It is impractical (and potentially discriminatory) to identify all patients who may be HIV infected. Precautions should be routine on all patients. Allergies Allergies, aka hypersensitivities, are an exaggerated immune response. In other words, it is where the reaction is to one’s own body tissues and is different than it should be. The reaction itself causes unwanted damage. This was at first thought to be a defense against intestinal parasites. Four types were classified in 1963. Type 1 and then 4 are the “allergic” disorders. It is unknown why some antigens evoke a type 1 response, and others the type 4. Type 1- Anaphylactic Reaction or Immediate Hypersensitivity This is an antibody mediated response. Reactions can range from localized allergic rhinitis or urticaria to systemic and life-threatening. o This is more common in kids, because their immune system is more advanced. Reaginic activity refers to an antigen that induces a Type 1 response immunologically. This can occur virtually immediately upon exposure to an antigen which has previously “sensitized” the host. Why some people will respond to some antigens with this type of reaction is unknown, but it is somehow polygenic. Desensitization o In prevention of systemic anaphylaxis, small doses of antigen are administered at 15 minute intervals (acute desensitization) to cause small scale formation of complexes and not a major reaction. Chronic desensitization involves the long-term administration at weekly intervals of the antigen to which the person is hypersensitive. This is an extremely dangerous process because of the possibility of inducing full anaphylaxis. o Allergy shots are designed to give minute amounts of antigen by a different route, and hopefully induce IgG as a blocking antibody. Types of Type I Reactions o Food allergies Chocolate, milk, nuts, eggs, shellfish, cheese, soybeans o Insect bites and bee stings o Hay Fever o Airborne allergens affect the upper respiratory tract Mold, dust, pollen Mast cells in the mucous membrane are sensitized (including eyelids, etc) o Ingestants, such as artificial colors or preservatives o Atopy Localized reactions that exhibit strong familial predisposition to hypersensitivity reactions and are associated with elevated IgE levels. The predisposition to atopy is genetic and symptoms are induced by exposure to the specific allergens typically found in the environment. o Drug Hypersensitivity (Oral or topical) Drugs, particularly antimicrobial agents like penicillin, are now amongst the most common causes of hypersensitivity reactions. The sequence of events is like as follows o First Exposure- Sensitivity Created Exposure binds to a specific antigen in a genetically predisposed person. B cells are converted to plasma cells via differentiation. The plasma cells form IgE (instead of IgG), called a reagin, which binds to mast cells and basophils everywhere, without the involvement of a complement meaning that there is no permanent damage to the tissue. The ability to produce this reagin is hereditary. o Second Exposure- Immediate Reaction Created On reexposure the antigen links onto the IgE on a mast cell. This causes explosive degranulation, causing histamine and other related substances to be released, triggering inflammation and smooth muscle contraction. Other effects include vasodilation, increased vascular permeability, and mucous secretion. Mast cells are found in most connective tissue. Factors released include Histamines: causes vasodilation, increased capillary permeability, and smooth muscle contraction. The bronchospasm so prominent in acute anaphylaxis us due in part to histamine release. Proteolytic enzymes Slow-reacting substance of anaphylaxis: principal mediators in the bronchoconstriction of asthma not influenced by antihistamines Eosinophil chemotactic factors of anaphylaxis: attracts eosinophils Serotonin Heparin Prostaglandins and thromboxane: prostaglandins cause dilation and increased permeability of capillaries and bronchoconstriction. Thromboxane aggregates platelets. If severe enough and system wide, then asthma, respiratory insufficiency, laryngeal edema, loss of intravascular volume, hypoxia, shock, and death can occur. Some of the reactions are localized, and some are systemic, therefore they are clinically different. o Systemic Effects A systemic reaction is due to the large number of mast cells and mediators located throughout the body. Respiratory impairment Smooth muscle impairment in bronchioles Arterioles Vasodilation to decrease blood pressure Increase permeability/ vascular collapse Fluid is lost and blood pressure decreases Anaphylactic shock due to decreased blood pressure, which can be life threatening. o Localized Effects (Atopy) Angioedema Urticaria (hives) Bronchospasm/ Bronchial asthma Involves lower respiratory system Possible to cause constriction of bronchioles and death. Tests o Intradermal skin test This involves applying a minute amount of suspected allergen via a superficial epidermal scratch or injection. A positive reaction is characterized by rapid onset (seconds or minutes) of wheal and flare. o Conjunctival Sac test This involves topically instilling a low concentration of the suspected allergen into the conjunctival sac. A positive reaction is demonstrated by immediate development of eyelid swelling, chemosis, conjunctival hyperemia, and itching. o These tests may cause anaphylaxis in highly susceptible individuals, and therefore extreme caution must be maintained throughout performing the tests. Type 2- Cytotoxic or Antibody-Dependent Hypersensitivity Here antibodies are formed against target antigens of the cell membrane, activating complement that damages those cells. Complement-mediated lysis or cytotoxin action causes cell and tissue damage. There are three different mechanisms involved in the Type 2 reaction o Complement-mediated cytotoxicity Here the antibody binds to the antigen component of the cell membrane and fixes complement, which leads to lysis of the cell. Examples are some hemolytic anemias, thrombocytopenia, and some kidney disease in the glomerulonephritis category. o Antibody-dependent cell mediated cytotoxicity Here target cells are coated with antibody and this somehow directly leads to cell lysis. This is not clearly understood, and no specific examples can be given. o Antibody-mediated cellular dysfunction This is the most common. Here antibodies attach to cell membrane surface receptors and interfere with cell function without damaging the cell itself. An example here is Myasthenia Gravis, where acetylcholine receptors are altered to impair neuromuscular transmission, therefore causing severe muscle weakness. This can also lead to stimulation of cells at the membrane receptor level and cause secretion by the cell, as in Grave’s disease. The immunoglobulin acts like TSH and increases thyroxin. Note that often a virus or a medication can be the environmental factor leading to the above polygenic reaction. In many cases, complement is involved, meaning that tissue is destroyed, resulting in permanent damage. The four tissues that are affected generally affected are the eyes, skin (especially face/eyelids), synovial Joints, and the kidney. Hemolytic anemias due to binding of drug to red blood cells, ABO transfusion reactions, organ transplant rejection, and Rh hemolytic diseases are also examples. Type 3- Immune-Complex Mediated Disease Here the problem is the combination of antigen/antibody complexes that leads to the formation of a very large molecule that tends to “lodge” in certain tissues. This initiates a severe, acute inflammation in tissues. Normally they are promptly removed by the reticuloendothelial system, but occasionally they persist and are deposited in tissues. This activates the complement system. The tissues mainly affected are the eyes, joints, skin, and kidney, but any tissue can be involved. o Arthus Reaction (localized): antigen, antibody, and complement are deposited in vessel walls; PMN cell infiltration and intravascular clumping of platelets then occur. These reactions can lead to vascular occlusion and necrosis. o The activation of complement is important in the PMNs and leads to severe inflammation. The PMNs contain enzymes to digest proteins. Examples o Serum Sickness o Immune-complex disease Glomerulonephritis Arthritis: serum and synovial fluid of rheumatoid arthritis patients contain “rheumatoid factor,” i.e., IgM and IgG antibodies that bind to the Fc fragment of normal human IgG. It is assumed that there are deposits of immune complexes on synovial membranes and in blood vessels that activate complement and attract PMNs to result in inflammation. Autoimmune diseases, like Lupus. Type 4- Delayed Hypersensitivity or Cell Mediated Hypersensitivity This reaction is mediated by T lymphocytes (CD4). In the misdirected immunity aspect, no complement is involved. The CD4 cells release lymphokines in the involved tissue, which leads to the severe inflammation. Unlike Type I hypersensitivity, in Type 4, tissue is destroyed. The sequence of events in misdirected immunity is as follows o The exposure to an antigen in a genetically predisposed person leads to sensitization, leading to a clone of T lymphocytes (CD4). The lymphocytes are a subset of T cells. They lead to exaggerated immune responses. The specific T cell is labeled as the Delayedtype hypersensitivity T cells or TDTH. These amplify inflammation. The lymphocytes react to antigens. Phagocytic cells are attracted and increase capillary permeability. o Reexposure leads to massing of the T lymphocytes in the area within hours to days and a severe allergic reaction follows due to release of chemicals in the area. The released lymphokines create an inflammatory reaction. The massing of cells means that phagocytic cells engulf particles, including tissue. o The response is delayed. It takes a couple of days to get the T cells activated. o A prime example is poison ivy, cosmetic, and metal allergies. This type of reaction is involved in the cell mediated rejection of transplanted tissue, lysis of viral infected cells, cancer rejection, and contact dermatitis. Patch testing on a small area of skin can sometimes identify the offending agent. Background History of exposure to new medications, new clothing, insecticides, new cosmetics, recent travel, gardening, or a new work environment (particularly industrial). Primary irritant dermatitis This is common and occurs upon initial exposure. It’s onset is within minutes. Delayed hypersensitivity reaction Prior exposure to sensitization to allergens. o Strong allergens need about 5-10 days o Weak allergens require months to years. Re-exposure to allergens. Reaction begins 12-72 hours later. This makes it difficult to determine the cause. These are mediated, not by antibody, but by T lymphocyte (cell-mediated immunity). Possible allergens Topical medications and DPAs. o “caine” preparations in topical anesthetics and antiitch preparations. o Neomycin, maxitrol, neosporin. o Bacitracin, BAK, thimerosal, atropine, timolol, phenylephrine, EDTA. Cosmetic preservatives o Perfumes and fragrances o Paraben mix (preservative) in cosmetics, topical creams, ung, and lotions, and topical corticosteroid preparations. o PPDA (paraphenylenediamine) found in permanent and semi-permanent hair dyes, PABA sunscreens, and sulfonamides. o Balsam of Peru found in perfumes, medications, and shampoo/hair products. o Creams, nail polish, and lanolin. Rubber, acrylics Plants Formaldehyde o In cosmetics, wash and wear clothing, and paper. Nickel o In many home and industrial products. Tests o Skin patch test A “band-aid” with known allergens is applied to a relatively hairless area of skin and covered for 48 hours to see if there is a reaction. Results are observed 24-40 hours after the cover is removed. Not uncommon to have false-positives and falsenegatives. o Usage test Actually use the suspected allergen and observe any subsequent reactions. o Blood test for allergies, aka Allergen Prick Test o Challenge Test- place the allergen against anti-allergens. Ocular Allergies Epidemiology At least 20% of the US population suffers from allergies, with 83% of these experiencing ocular symptoms. The two main symptoms reported are itchy and watery eyes. The ocular allergies correlate to a diminished quality of life. Pathophysiology and Classification Ocular allergies are ocular manifestations of a systemic allergic immune response. The most important cell in an allergic response is the mast cell, which initiates the entire allergic response. There are about 50 million mast cells in the ocular tissue of each eye. This is about 5000 per cubic mm. Allergens attract and adhere to the IgE molecule on the mast cell surface. An influx of calcium i ons causes the movement of granules toward the cell membrane and the release of preformed and later newly-formed chemical mediators. o Preformed mediators These are available to react immediately with tissues. Examples include histamine, proteoglycan (heparin), neutral proteases such as chymase and tryptase, and platelet activation factor. Histamine and bradykinin immediately begin to stimulate nerve endings (nociceptors), creating the sensation of itching for roughly 30-60 minutes. They both also increase vascular permeability and vasodilation, causing the clinical signs of redness and conjunctival injection. o Histamine produces its effects by binding to histamine receptors of the H1 and H2 types. Chymase causes mucin secretion and increases vascular permeability. Tryptase leads to the production of proinflammatory prostaglandins and fibroblast proliferation. Heparin, an anticoagulant, promotes fluid leakage from blood vessels. Other mediators attract RBCs and WBCs to the area, and these cells easily reach the conjunctival surface by moving through the dilated and highly permeable capillaries. o Newly Formed mediators These are formed after the mast cell degranulates. Phospholipase A2 acts directly on the phospholipids contained in the mast cell’s outer membrane, forming prostaglandin and arachidonic acid. Any disruption or threat signals the cells to convert phospholipids into arachidonic acid. During the inflammatory reaction, two enzymes, lipoxygenase and cyclooxygenase, further metabolize arachidonic acid further producing other newly formed chemical mediators. Arachidonic acid interacts with two enzymes, cyclooxygenase and lipoxygenase, metabolizing it into three newly formed inflammatory mediators which are collectively known as eicosanoids. Prostaglandins Thromboxanes Leukotrienes An allergen’s presence initiates the arachidonic “cascade” both within conjunctival epithelial cells and also within mast cells as they degranulate. Much like histamine and bradykinin, prostaglandins directly stimulate nerve endings to produce sensations of itching and pain, lasting 4-8 hours. Prostaglandins also increase vascular permeability and vasodilation Leukotrienes primarily attract WBCs (macrophages) o Decreased phospholipase starts the inflammatory cascade. Summary Exam History is critical. o What was the onset time? Sudden? Over a period of time? Seasonal? Year round? Generally there is a sudden, definitive onset. Ask for specific contacts. One needs to specify the allergen and avoid them. Onset is generally seconds to minutes after exposure. Are there any other allergy signs? Rhinitis? Has there been a lifestyle change? New pet, house, clothes? o Family history o Symptoms and signs Ocular and systemic Vision may fluctuate or be unchanged. Types Allergic Lid Disease (373.32) Atopic Blepharitis (373.31) (Lost in Editing?????)- See lids notes. Type I Allergic Reaction o The causes and more information on this is listed earlier in the immunology section. o Ocular allergic reactions include seasonal allergic conjunctivitis, atopic keratoconjunctivitis, vernal keratoconjunctivitis, and drug exposure. o Ocular signs and symptoms The eyelid is very thin, therefore it is highly sensitive to allergens. This vascularized, spongy tissue is capable of edema and swelling Urticaria/ Hives Smooth, slightly elevated patches (wheals) Redder than or paler than surrounding skin. Generally there is a blanched center with an erythematous margin. The underlying tissue is normal and not swollen. It is warm, tight, and one can see individual pores. Angiogenic edema This is a higher risk of anaphylaxis. Superficial skin (epidermis) is normal and the deeper tissue edematous. Lid(s) are swollen producing either a ptotis or closed lid. Itching This is 100% necessary. On a scale of 1-10, 10 beingthe worst, patients will almost always describe this as a 8-10. This occurs soon after contact with some topical antigen, suggesting an IgE Type I immediate hypersensitivity. Rarely lasts more than several days. Type IV Allergic Reaction- Contact Dermatitis (372.22) o The causes and more information on this is listed earlier in the immunology section. o Ocular allergic reactions include giant papillary conjunctivitis, herpes simplex virus, toxoplasmosis, phylectenulosis, and contact dermatitis. o Ocular signs and symptoms Itchy, watery, teary eyes Eyelids A rash and/or swelling occurs on and/or around the eyelids. There is slight eyelid edema with mild scaly eruptions and erythema. o Early this appears as oozing, erythmatous skin, in which the skin dries, thickens, dry or wet scaly peeling (eczema) during the chronic state. Dennie-Morgan folds o This is a linear fold of skin that develops below the lower lid secondary to edema. Conjunctival involvement Papillary reaction, hyperemia, and chemosis. Seasonal and Perennial Allergic (Hayfever) Conjunctivitis (SAC/PAC) (372.11) o Background This is an allergic response of the body’s immune system to foreign substances known as allergens which the body perceives as a potential threat. The response can be innate or adaptive. This is a very common ocular irritation. It represents 95% of all ocular allergies. This form of conjunctivitis, along with viral, is more common than bacterial. PAC is a year-round problem with milder symptoms than SAC. It tends to be associated with asthma and caused by ever-present, often indoor, allergens. In pediatric patients, PAC often is a clue that leads to an asthma diagnosis. If SAC is left untreated, it can progress to a chronic inflammatory condition characterized by chemosis, hyperemia, and lid edema. It occurs more frequently in atopic patients, those with a history of hay fever, atopic dermatitis, allergic rhinitis, and asthma. It typically affects those in the second decade of life. Atopy is generally genetic. A child who has one atopic parent is 4 times more likely to develop allergies (33%), and a child with two atopic parents is 10 times more likely (70%). o Causes PAC SAC Animal dander Airborne dust Dust mites o The average bed contains more than 10,000 dust mites and more than 2 million droppings. The average pillow doubles in weight in 6 months due to the droppings of the house dust mite. Airborne mold, spores, fungi, year round or seasonal. Plant matter, varies with season. Perhaps the likeliest cause. o Tree pollens in spring o Grasses (timothy, blue grass) in summer o Weeds (ragweed, sage) also molds in autumn. Also some delayed hypersensitivity allergies; usually lid or periorbital tissue involved rather than conjunctiva, with comparatively minimal conjunctival hyperemia. With this, the problems surface, meds are given and the patient gets better, then worse. Do not prescribe more meds! An rx is generally just used to alleviate the symptoms. o Exam 99212 o Signs Bilateral red, watery, itchy eyes. Lids Mild papillary hypertrophy on upper lid. Mild inferior papillary reaction. No follicles are seen on the inferior fornix. Lid tissue displaying signs of allergy. o Lid edema, angioedema, uticaria Wheals due to venule distention. o Weeping, eczematous reaction (contact dermatitis) o Excoriated, scaly (atopic dermatitis) o Extra lid fold beneath lower lid margin (DennieMorgan fold) due to edema. Long lashes are seen in these patients “for protection.” Discharge Possibly watery (acute onset) or thin, ropy, almost transparent mucoid discharge (chronic, seasonal). Conjunctiva Chemosis with glassy/gelatinous appearance of conjunctiva. This may be so severe as to drape or bulge over the lid margin or limbus. Hyperemia often less severe than chemosis, but is exacerbated by eye rubbing. This is due to the fact that it breaks down mast cells releasing histamine. It may be worse in the eye associated with the dominant hand. Corneas are clear. Perhaps a mild punctuate keratitis on the inferior cornea. This is not sight threatening. No PAN Clinical signs include fewer eosinophils in scrapings, possible spikes in tear histamine, but normal histaminase function. o Symptoms Itching (possibly intense or unrelenting). This is generally the main complaint, along with watery, tearing, red eyes. Foreign body sensation, worse in the morning, from chemosis. Lids possibly matted and/or swollen in the morning. Atopic Keratoconjunctivitis (372.05) o Patients must have eczema to have AKC, which is a Type IV response. Patients are genetically predisposed to this. o Generally the patients are older, but can occur as early teen years. o Signs and symptoms include itching, redness, photophobia, keratopathy, ulcers, keratoconus, anterior polar cataracts, mucous discharge, and atopic blepharitis. They present with eyelid thickening, skin changes in the upper lids and sometimes in the lower lids, giving them a raccoon-like appearance. The skin around the eyes is dry and itchy. In severe cases there can be loss of eyelashes, conjunctival or corneal scarring, and watery eyes or mucous secretions. Clinically, these patients have elevated levels of eosinophils and mast cells. The symptoms arise between the ages of 20-50. o These can involve the cornea and threaten vision. o Should have allergy testing done. Vernal Conjunctivitis (372.12) o Epidemiology This is a severe, chronic type I allergic condition affecting the conjunctiva and cornea. The first attack is generally the worst. o o o o It usually affects children and young adults with a personal or familial history of allergies. It is rarely seen in kids less than 3 years of age, and rarely older than 25. It is usually developed by 14 years or younger. The disease has a duration of 4-10 years. It is seen more in younger males 2:1 early on, with the gender predilection equalizing later on. There is a greater prevalence in those with a strong personal or family history of atopy, including asthma, allergic rhinitis, eczema, and hay fever. This recurs during the spring and summer months and in warmer, subtropical/tropical climates. It can recur anytime of the year and even persist year-round for some individuals. Symptoms are generally worse during the warmer months if year-round. Pathophysiology Can be genetic atopy, abnormal eosinophil activity, SAC, PAN, or a nonspecific hypersensitivity. Two forms of VKC are seen. Palpebral VKC The inflammation is located predominantly on the palpebral conjunctiva surfaces, especially the upper tarsus, where a diffuse papillary hypertrophy can develop into giant cobblestone palpebral papillae. Limbal VKC The inflammation is located predominantly on the limbus, especially the superior limbus (more common in black and Asian populations), where the limbus has a thickened gelatinous appearance lookling like large, sausage shaped limbal papillae. Occasionally both palpebral and limbal VKC are observed in the same patient. Exam 99203 Signs Lids and Conjunctiva The eyelids may be swollen and red. Swelling of lids may cause a ptosis. Hyperemia and papillary hypertrophy of the superior palpebral conjunctiva. Smaller papillae are soon replaced by “giant cobblestones.” Papillae may form at the corneal limbus due to the high amounts of vascularization. As the papillae accumulate together, a milky veil forms over the top. This is best seen with Lissamine staining. There is also a pooling in between the papillae of NaFl. Mild inferior papillary reaction. Bilateral, recurrent inflammation of the superior palpebral and limbal conjunctiva. Horner-Trantas dots o These are active and calcified eosinophils. These are seen in atopic diseases next to the limbus on the bulbar conjunctiva. o This is more common in blacks and American Indians. Cornea A diffuse punctate epithelial keratopathy is seen, especially on the superior cornea. This stains with NaFl. o Diffuse white “flour-dusting” corneal SPK This is called Keratitis of Togby. o Filamentary keratitis is possibly present. o This can decrease VA, as well as causing foreign body sensation and photophobia. Shield ulcers o These are very uncommon. They are well-delineated sterile ulcers, generally on the mid-superior cornea. It is an oval shape with underlying stromal opacification. o This is due to the mechanical sloughing and mucoid discharge creating hydrophobic areas. o This is more seen in cases of palpebral VKC, and they are difficult to treat. Consider a referral to a corneal specialist. Neovascularization Clinically see increased levels of superficial mast cells, eosinophils, and lymphocytes and decreased levels of histamine. (-) PAN, since there is no infection. Condition may have exacerbations and remissions over several years o Symptoms This is a disabling condition for the patient and family. Patient is often thinking of discontinuing contact lenses. Itching is the primary symptom, followed by foreign body sensation. This is exacerbated by exposure to dust, wind, bright lights, pollen, or physical exertion. Some patients may describe this as a burning. These symptoms may be severe. Blepharospasm due to itching. Photophobia; very severe, even in dim light; disabling. This is due to the corneal breakdown and inflammatory mediators. Irritation, discomfort, red, hot swollen eyelids Chronically removing visually disabling thick, profuse, tenacious, ropy mucous strands; often visual blurring. These are worse in the morning. Known chronic allergies Intermittent eczema. Giant Papillary Conjunctivitis o Background Seen more often with CL wearers, especially hydrogel. It is a papillary response of the upper palpebral conjunctiva. It is caused by mechanical irritation, which can come from contact lenses, exposed sutures, extruded scleral buckles, ocular prosthesis, cyanoacrylate adhesives, and ocular foreign bodies. It can aggravate or be aggravated by other comitant ocular allergies. o Symptoms Patient complains of itching, fluctuating vision, stringy discharge, and loose lenses. Irritation, CL discomfort No chronic allergies Noncompliant with cleaning of CL. \ o Signs Multiple protein deposits on center of CL Clear lid margins Cobblestones Severe nonstaining papillary hypertrophy Moderate hyperemia of the superior palpebral conjunctiva Moderate mucous strands in the tear fil, and upper fornices Mildly hyperemic bulbar conjunctiva Clear corneas Treatment Patients Education o This is a chronic condition with no one-time cure. Identify specific allergen(s) by history. This can be difficult to identify, and avoid known allergens. o If bee sting, flick out stinger if present; do not tweeze since venom is forced out by tweezing Compresses o Cool These should be performed every 20-25 minutes or as often as needed, especially for the first 24 hours. These stop vessel leakage and reduce the buildup of edema. Vasoconstriction reduces chemosis. It is definitely indicated for allergies. This works best if the cloth is frozen or iced. Face cloth under cool water is not the best. o Warm These should be used for the 2nd and subsequent days to enhance the absorption of fluid. Warm compresses may assist in resolution of chemosis by vasodilation, but will likely exacerbate the irritation o Patient should experiment with alternating cool and warm compresses; cool compresses will provide immediate relief and will likely be preferable. Skin Treatments o Astringent (Burow’s) if the skin isn’t too dry. This is used only in acute presentations. Domeboro powder packets 1:40 or 1:20 (OTC) Soak clean cotton cloth in solution (old t-shirts, bedsheets, pillow cases) and soak affected area for 20 minutes bid to qid. Soaking dries the skin and restores normal texture; do not use when the condition is chronic. Reassurance Antihistamines o Agents Oral antihistamines These are best to use if there are related systemic symptoms, ie, rhinitis, wheezing, and dermatitis. They are also great to alleviate itching. Type IV does not respond as well to antihistamines. Agents o OTC Chlorpheniramine maleate (Chlor-Trimeton) 4mg, po, qid Diphenhydramine (Benadryl) 25-50mg, po, tid to qid. Clemastine (Tavist) o Rx Loratadine (Claritin) 10mg, qd Cetirizine (Zyrtec) 5-20mg, qd Fexofenadine (Allegra) 60, 100, 500 mg bid Desloratidine (Clarinex) 5mg qd Hydroxyzine (Atarax or Vistaril). This is the strongest and is available by prescription only. Antihistamine drops H1-Antagonist drops decrease the itching and redness. With these, there is no effect on mast cells, so it is an excellent medication, but only for short-term. Use three times a day. Agents o Levocabastine hydrochloride 0.05% (Livostin) o Emadastine difumarate (Emadine) o Mechanisms These compete with histamine at histamine receptor site. Antihistamines and vasoconstrictors may work synergistically. The response and improvement is idiosyncratic. Adverse effects Medication induced dryness can further irritate eyes that are already suffering the effects of ocular allergies. Allergic responses are exacerbated in dry eyes because low tear volume inhibits washout of allergens, prolonging the contact time with the conjunctiva. Decongestants o These reduce serous leakage. These are OTC, so they are much cheaper. Best used for rhinoconjunctivitis, not conjunctivitis alone. It is way too aggressive. o Topical vasoconstrictors These are generally not the best to use. Agents Oxymetazoline (Ocuclear) Phenylephrine (Prefin)- now discontinued Naphazoline (Degest 2, Albalon) Tetrahydrozoline (Visine) o Oral decongestants Pseudoephedrine 30, 60mg / 120, 240 mg extended release (Sudafed OTC) Phenylpropanolamine (Triaminic) o Oral antihistamine + Decongestant Prophylaxis with antihistamine is more effective than symptom relief. Agents Loratadine 5mg + pseudoephedrine 120 mg (Claritin-D Rx) Chlorpheniramine 4mg + pseudoephedrine 60 mg (Sudafed Plus) OTC Eye drops o Pheniramine with naphazoline Naphazoline concentration in combo products is stronger than in OTC naphazoline decongestants. Combo has 0.025-0.05% vs. 0.012-0.02% in noncombinations. Naphcon-A (0.3% pheniramine, 0.025% naph) Opcon-A (0.315% pheniramine, 0.025% naph) o Antazoline with naphazoline Vasocon-A (0.5% antazoline, 0.05% naph) o These are not good on a chronic basis due to the preservatives that are used. Mast Cell Stabilizers- prevent histamine release from mast cells. o Problem with these is that is takes about 12-14 days to take effect. This is difficult with compliance. It is poor for acute relief; best for prophylaxis for seasonal sufferer starting prior to symptoms. It is better at reducing symptoms than at improving clinical signs. o Cromolyn sodium nasal spray (Nasalcrom) for allergic rhinitis o Cromolyn sodium 4% gtt (Crolom solution/Opticrom) for allergic conjunctivitis currently recommended only for vernal keratoconjunctivtis. Qid for 1mo, tid for 1 mo, then BID. This can be used longterm. o Iodoxamide (Alomide solution) is currently recommended only for vernal conjunctivitis. This is about 1000x more potent, so it is the best. o Nedocromil sodium (Alocril) bid, has a possible side effect of HA due to the induced vasoconstriction. o Alamast (pemirolast potassium) 0.1% Combination mast cell stabilizer/anti-histamine- bid; expensive o Patanol (olopatadine, 0.1%) 0.2% Pataday. Once a day dosing. o Zaditor (ketotifen fumarate) 0.025% o Optivar (azelestine) 0.05% o Epinastine (Elestat) 0.05% This has the strongest affinity for both H1 and H2 receptors. This also interacts poorly with muscarinic receptors, theoretically having the lowest potential to induce ocular drying. It exhibits the highest antihistaminic potency and lowest antimuscarinic activity. Topical steroids- tid-qid o Generally indicated for severe conditions (vernal conjunctivitis) and type IV. It decreases chemosis, inflammation, and itching. Do not use them chronically (greater than 1 month). Use only when appropriate. Provides a quick fix for minor allergies. o Prone to overuse by the patient. Dispense a small bottle (2ml) when possible. Prone to overprescribing by the doctor. o Danger of masking infections. This can actually increase the risk of infections and increase IOP. o Prednisolone acetate 0.12% (Pred Mild), fluoromethalone 0.1% and 0.25% (FML, FML Forte) are all suspensions; may be uncomfortable since slightly gritty. Doesn’t penetrate the cornea as well. o Prednisolone sodium phosphate 0.12% (INflamase Mild) is a solution, somewhat more comfortable and with less corneal penetration. o HMS 1% (medrysone) is weakest, but also a suspension. o Alrex (loteprednol efabonate 0.2%)- steroid FDA approved for treatment of ocular allergies qid for 1-2 weeks. Generally used qid 3-4 days then taper over 2 weeks. This is the safest for longterm use. Still has risks. o Allergic conjunctivitis from delayed hypersensitivity (to atropine or neomaycin) requires steroid-pulse q2-3h for 2-4 days, then quickly taper off; use pred sodium phosphate 1% (INflamase Forte), FML, or FML Forte or Alrex o Topical dexamethasone ointment for quickest relief of symptoms and signs. o Topical cortisone cream or ointment to calm itching, bid OTC 0.5% to 1.0% hydrocortisone products Lotion (humid) or ung (dry climate) Anti-itch lotions are possible, though they run the risk of excessive drying: calamine lotion, Caladryl (calamine plus benadryl) o Use of steroids Consecutive regimen attempts to keep patient off steroids as much as possible. o Keep at q2h for full duration of pulse or cut to q4h after 3 days at q2h. o “7day rule”- can stop steroids “cold turkey” at 7 days without needing to taper. o Provides relief without completely eliminating symptoms; patient should attempt to tolerate some discomfort. o Provide relief with topical NSAID or topical antihistamine after steroid pulse. Longer use of steroids o Keep at q2h for full duration of pulse. Consider FML. o Slow taper of steroids; begin after one week pulse, then reach lowest maintenance dose which prevents rebound of allergy; stay at this maintenance dose for 4-6 weeks. o Using less than the maintenance dose will result in exacerbation of condition. o After 4-6 weeks, attempt to further taper off the steroid to zero drops. o This disease is hard to use steroids with since it is long term, and you do not want to use steroids long term. Always check intraocular pressures before and during steroid therapy; consider FML, Vexol, and Lotemax drugs with family history of glaucoma or for a steroid responder; most elevated IOP elevations require 2-3 weeks and may not present during the one week pulse; critical to avoid iatrogenic effects of steroids. As an alternative to maintenance does, some suggest a “steroid allowance” of a maximum of 10 drops per week; use as few drops as possible if symptoms are well controlled by other means. o Corticosteroids for short-term symptomatic relief Pulse therapy- 1gt Pred acetate 1% q2h during waking hours x3dy, then qid for 3-4day, then discontinue 0.1% fluorometholone acetate (Flarex) or 0.5% loteprednol (Lotemax) appears to be an excellent alternative to 1% prednisolone. Strong steroid pulse when symptoms are 2/3 reduced Significant danger from over-utilization; encourage patient to tolerate some discomfort. Goal is to provide some relief, not totally eliminate the symptoms. Long-term, high frequency use of steroids should be avoided at all costs. Hopefully MCS with all adjunctive therapies will provide enough relief so that steroid pulses will be needed infrequently. Repeat at next flare up. May require 1-3 treatments during the season. Topical NSAIDs- qid o NSAID Drops Ketorolac (Acular). These drops have significant benefit. Diclofenac (Voltaren Ophthalmic); may be used in future for allergy Flurbiprofen (Ocufen) and Suprofen (Profenal), may be used in future for allergy. o NSAID ointments Elidel (pimecrolimus) Protopic (tacrolimus) Off-label leukotrienes (cingulair, accolade) Very severe cases (poison oak, etc) may require systemic steroids, usually po or IM x 1week. If angioedema is due to a medication allergy, this could be significantly more serious than a bug bite. Options include epinephrine injection and/or calling 911. Desensitization by serial administration. Antibiotics o Essential to treat any infection first. Follow-up o Severe condition: see the next day o Moderate condition: see in 2-3 days o Mild condition: see in 3-5 days or PRN. o Type IV expect much slower resolution for chronic (eczematous) type. Medication usage. o During an attack, patients should use the medication until their symptoms resolve, and then stop. Unless it is PAC. o AT can help keep dry eye symptoms at bay for many patients and offer some allergy relief for minor nonacute episodes. With no improvement of symptoms, treat for dry eye. o Treat concurrent dry eyes. Contact lenses include increased risk of developing ocular dryness and irritation. Treatment for Atopic Keratoconjunctivitis/ Type IV Reaction o Acute presentation Oral antihistamine, OTC or Rx Topical steroid Oral steroids (prednisone) only in very severe cases. 20mg po bid. o Subacute/ Chronic presentation Skin moisturizer such as Lubriderm, etc. (No astringents, because the skin is already dry) Topical cortisone ointment or lotion. Antiitch creams or lotions: Caladryl. This may accentuate dryness but relieve the itching. Topical steroid, oral antihistamine possibly Risk of lichenification, with resulting excoriation and secondary infection. o Conjunctivitis (from atropine, neomycin) usually requires steroid pulse with prednisolone 1% or FML, short-term. o RTC 2-4 weeks. Compliance is generally not an issue since the medication gives patients relief from the symptoms. o Ophthalmic ointment for thick, leathery, scaly lid changes. Dexamethasone ointment, or tobradex ointment if steroids are not available. OTC steroids are not the best, because they can thin the eyelid tissues. Treatment for VKC o Mild Cases Cool compresses and artificial tears can be used as much as needed, at least qid. Environmental changes should be made, such as home air conditioning, relocation to a cool environment, hepa filters, indoor sunglasses to decrease photophobia and block allergens, etc. Topical vasoconstrictors and antihistamines, qid Oral vasoconstrictor-antihistamines bid to qid, though topical therapy is much more effective than oral. o More Severe Cases Mast cell stabilizers If possible, anticipate the start of vernal conjunctivitis season by 3-4 weeks for “experienced” VKC sufferers. These drops often have a “lag” period of 3 weeks, though many patients experience relief sooner. Regimen is qid, ou. Decrease this to tid/bid after one month. Iodoxamide (Alomide) is the best. Cromolyn (Crolom) indicated for VKC also. Up to 3 months of use is allowed. This is extremely valuable management since it provides significant relief. The first episode of VKC will need MCS with a topical steroid pulse (with taper), and all adjunctive therapies. Steroids A pulse steroid is the preferred mode of steroid use. This is a high amount, in a decreased amount of time. o Start q1h for 5-7days, then q2-3h the next 3-5 days. Q4h then next 5-7 days. One must judge how it is working. A strong steroid is used (1% prednisolone acetate or sodium phosphate; 0.1% fluorometholone acetate or 0.25% fluoromethalone alcohol, Lotemax) Limbal vernal or milder forms of upper lid vernal probably do not require maintenance doses of steroids. o In the presence of an ulcer Polytrim gtt q4h in conjunction with the above therapies if shield ulcer is present. Antibiotics could also be used prophylactically. o Non-approved therapeutic modalities Oral aspirin (650mg, tip, po) This is very effective on VKC, but tricky due to GI upset in kids, as well as possibility of Reyes syndrome. Topical NSAIDs- not as great Anecdotal cases suggest this is very effective for patients when used for one week (per manufacturer) Catania says topical NSAIDs do not work as either initial therapy or as a substitute for maintenance steroid therapy due to loss of effect after about 30 days of use. Acetylcysteine 10% (Mucomyst) qid to reduce mucous. 2% cyclosporine A gtt (Restasis). This is very effective for kids, but it is slow (45 days). Once the drops are discontinued, the disease usually recurs. Olopatadine (Patanol), ketotifen (Zaditor), azelastine (Optivar)unknown effects on VKC at present. Ketorolac (Acular)- approved for SAC. o Refer to an allergist for atopy and a dermatologist for eczema. o Regular follow-up Risk of shield ulcer, corneal scarring Superior corneal SPK, vulnerable to pathogens Minimize risks by thorough management of any lid disease. Treatment of GPC o Since the allergen here is generally known, the allergen should be removed. Discontinue CL for 3-4 weeks. Mild GPC can be resolved with discontinuation of CL wear alone. Frequent replacement of CL or use of disposable lenses is also effective in reducing the chance of developing GPC or reducing mild GPC. o For Milder Cases Antihistamine /MCS combos Topical antihistamine-vasoconstrictor combinations used q4h are usually effective in providing relief of symptoms in more advanced cases. o For More Severe Cases 1-2% mucomyst q4h is effective in reducing mucous discharge, but is not FDA approved for ophthalmic use. Suprofen 1% (Profenal) Loteprednol etabonate 0.5% (Lotemax) q8h o May require more frequent office visits to monitor stabilization Immunological Tests Diagnostic Antigen-Antibody Reactions o Agglutination: The agglutination is particulate (eg, bacteria and red blood cells) or is an inert particle (latex beads) coated with an antigen. Antibody, because it is divalent or multivalent, cross-links the antigenically multivalent particles and forms a latticework, and clumping (agglutination) can be seen. o Precipitation: The antigen is in solution. The antibody cross-links antigen molecules in variable proportion and aggregates (precipitates) form. In the zone of equivalence, optimal proportions of antigen and antibody combine. The maximal amount of precipitates forms, and the supernatant contains neither an excess of antibody not an excess of antigen. In the zone of antibody excess, there is too much antibody for efficient lattice formation, and precipitation is less than minimal. In the zone of antigen excess, all antibody has combined but precipitation is reduced because many antigen-antibody complexes are too small to precipitate. Radioimmunoassay (RIA) o This method is used for the quantization of antigens or haptens that can be radioactively labeled. It is based on the competition for specific antibody between the labeled (known) and the unlabeled (unknown) concentration of material. The complexes that form between the antigen and antibody can then be separated and the amount of radioactivity measured. The more unlabeled antigen present, the less radioactivity there is in the complex. The concentration of the unknown (unlabeled antigen or hapten is determined by comparison with the effect of standards). RIA is a highly sensitive method and is commonly used to assay hormones or drugs in serum. The RAST (radioallergosorbent test) is a specialized RIA that is used to measure the amount of serum IgE antibody that reacts with a known allergen (antigen). Enzyme-linked Immunosorbent Assay (ELISA) o This method depends on the conjugation of an enzyme to either an antigen or an antibody. The enzyme is detected by assaying for enzyme activity with its substrate. o It detects systemic infection of inflammation by measuring the rate of fall of red blood cells. In infection or inflammation, acute phase reactants will cause the rate of fall to increase. This test is useful for temporal arthritis or polymyalgia rheumatica. Immunofluorescence o Fluorescent dyes can be covalently attached to antibody molecules and made visible to UV light in the fluoresecent microscope. Such labeled antibody can be used to identify antigens, eg on the surface of bacteria, in cells in histological sections, or in other specimens. Complement Fixation o The complement system consists of twenty or more plasma proteins that interact with one another and with cell membranes. Each protein component must be activated sequentially under appropriate conditions for the reaction to progress. Antigen-antibody complexes are among the activators, and the complement fixation test can be used to identify one of them if the other is known. Complement fixation takes place in two stages In the first stage, if the antigen and antibody are homologous, complement is fixed to the antigen-antibody complex. In the second stage, sensitized red blood cells are added. If complement was fixed in the first stage, less complement remains free to lyse the red cells. Therefore, lysis of red cells is reduced. HLA-B27 (Human Leukocyte Antigen) o This test is used to diagnose predisposition of a person to rheumatoid spondylitis. Tissue Biopsy o This uses tissue material to determine the presence of disease such as temporal arteritis. Antinuclear Antibody o This test is used to diagnose antibody to nuclear proteins in testing for collagen disease such as SLE. Acetylcholine Receptor Antibody o This test is used to diagnose antibody to acetylcholine receptor in myasthenia gravis. Flow Cytometry of B and T cells o This test is used to diagnose immunodeficiency in diseases like AIDS and chronic lymphocytic leukemia Neutralization Tests Immune Complexes Hemaglutination Tests Antiglobin Test Antigen-antibody reactions involving red blood cell antigens o ABO blood groups and transfusion reactions o Rh blood type and hemolytic disease of the newborn Immunopharmacology Immunosuppressants These are more specific than steroids and NSAIDs. They are agents that reduce the body’s response to antigenic stimuli. Adverse effects of immunosuppressants in general include increased susceptibility to infection and cancer. Glucocorticoids- Prednisone o Mechanisms Decreases IL-1 production from macrophages Decreased expression of MHC class II antigens Decreases phagocytosis o Uses Treat autoimmune disorders and prevent graft rejection Cytotoxic agents o Cyclophosphamide (Cytoxan) Mechanism- Blockade of lymphocyte proliferation; a metabolite alkylates DNA Humoral immune responses are more sensitive than cell-mediated responses Routes of administration Oral or IV Uses Autoimmune disorders, Prevent graft rejection. , Necrotizing scleritis, Cicatricial pemphigoid, Behcet’s syndrome, Mooren’s ulcer, Ocular manifestations of RA, Ocular manifestations of Wegener’s granulomatosis, and Cancer Adverse effects Alopecia, nausea, vomiting o Chlorambucil (Leukeran) Mechanism- Blockade of lymphocyte proliferation by alkylating DNA Route of administration- Oral Use- Behcet’s syndrome Adverse effects Bone marrow depression, Sterility, and Teratogenesis o Methotrexate Mechanism of action Competes with dihydrofolate for the enzyme dihydrofolate reductase. Less tetrahydrofolate is formed, creating a deficiency of methyl groups available for synthesis of purines and pyrimidines by thymidylate synthase. This blocks lymphocyte proliferation. Use Steroid-resistant uveitis, cancer, autoimmune disorders Route of administration- Oral Adverse effects Systemic- Bone marrow depression, GI upset, hepatic cirrhosis, and teratogenesis Ocular- Irritation, tearing, photophobia, and aggravation of seborrheic blepharitis. Contraindications Pregnancy Lactation Chronic hepatic disease Immunodeficiency syndromes o Azathioprine (Imuran) Mechanism of action Metabolized to 6-mercaptopurine, then to thioinosinic acid Interferes with purine metabolism (and therefore nucleic acid synthesis) during lymphoid cell proliferation following antigen stimulation. Cell-mediated immune responses are more sensitive than humoral responses Uses- Used in conjunction with steroids when steroids alone are not sufficiently effective or are excessively toxic. Prevent graft rejection. Adverse effects Bone marrow suppression, nausea, vomiting, anemia, and thrombocytopenia Contraindications Pregnancy Treatment with other immunosuppressants Allopurinol o Colchicine Mechanisms Binds to tubulin, interfering with the function of mitotic spindles, so cells cannot combine. Inhibits migration of granulocytes to inflammatory sites Inhibits phagocytosis and axonal transport Inhibits release from granules of chemical mediators in secretory cells. Use- Behcet’s syndrome Route of administration- Oral Adverse effects GI upset, bone marrow depression, decreased fertility in males, and teratogenesis Immune Modulators o Block lymphokine synthesis or inflammatory effects of the immune response. o Cyclosporine A (Sandimmune) Mechanism- Blockade of cytokine production (Interleukin-2, Interleukin-4, Interferon gamma, and Tumor necrosis factor) Routes of administration Oral Topical- solution and 10% ointment (lipid soluble) Uses Autoimmune disease Prevent graft rejection Endogenous uveitis Behcet’s syndrome Sympathetic ophthalmia Corneal graft rejection Severe chronic vernal keratoconjunctivitis Paracentral corneal ulcers. Most of these utilize cyclosporine once steroids have failed. Adverse effects Systemic administration o Renal damage, hypertension, gingival hyperplasia, hypertrichosis, hepatic damage, paresthesia, GI upset. o No bone marrow depression. Topical administration o Transient superficial punctuate keratitis and mild ocular congestion, but these are reversible if discontinued. Contraindications Allergy, lactation, renal disease. o Dapsone Mechanism- Unknown Use- Cicatricial pemphigoid and leprosy Route of administration- Oral Adverse effects Hemolytic anemia and GI upset Contraindications Allergy (if allergic to sulfonamides) G-6-PD deficiency Lactation o Bromocriptine (Parlodel) Mechanism- Possibly suppresses prolactin release Use- Severe endogenous uveitis Route of administration- Oral Adverse effects GI upset, headache, dizziness, and fatigue Contraindications Allergy to ergot alkaloids Ischemic heart disease, postural hypertension Peripheral vascular disease Pregnancy Immunostimulants Interleukin-2 o Mechanism- Stimulates proliferation of T-helper cells and CTL cells o Use- Cancer o Adverse effect- Edema Colony-stimulating Factors o Multi-CSF (Interleukin-3) Stimulates progenitor cells of granulocytes, megakaryocytes, mast cells, macrophages, and erythrocytes o G-CSF- Stimulates progenitor cells of granulocytes o Cilmostim (M-CSF, Macstim)- Stimulates progenitor cells of macrophages o GM-CSF- Stimulates progenitor cells of granulocytes and of macrophages o Use- Treat bone marrow depression Interferons o Interfere with syntheses of proteins and RNA o Alpha-IFN- Useful in treating leukemias o Gamma-IFN- Induces MHC expression on macrophages and B cells o IFN-Beta- Treats MS