Limiting and Excess Reagents
advertisement

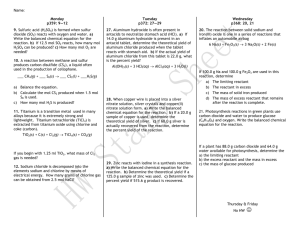
p.10-11Limiting and Excess Reactants/Reagents Reaction between 2.50 g of aluminum and 375 mL of 0.10 M copper (II) sulphate pentahydrate solution 1.a) Write a balanced equation of this reaction 2Al(s) +3CuSO4•5H2O(aq) Al2(SO4)3(s) +3Cu(s)+15H2O(l)+ energy 2.50g 375 mL, 010 M b) Write the stoichiometric ratio 2 mol 3 mol 1 mol 3 mol 15 mol c) n(Al) = 2.50 g X mol = 0.0926 mol Al 27.0 g n(CuSO4•5H2O) = 0.10 mol X 375 mL X 1 L = 0.0’37’5 mol L 1000 mL d) Predict the limiting and excess reactants: limiting: reactant is completely used up at the end of the rxn excess(xs): reactant is left over reactant at the end of the rxn limiting: CuSO4•5H2O used up excess: Al left over e) Write 3 chemical changes colour change: turquoise to greyish colour red solid is formed (Cu) temperature is increased (exothermic) 2. How can we find the limiting reactant? In order to find the limiting reactant, we do the following: Road Map: n(each reactant) n(one product) mass(one product) The keeper (limiting reactant) is: the reactant producing the least amount of product 3. Road map: p.11 mole Have bridge X MM Want n(reactants) ----- n(Cu) ----- xg(Cu) (product) (i) mass of copper produced from aluminum: xg(Cu) = 0.0926 mol Al X 3 mol Cu X 63.5 g = 8.82 g Cu reactant 2 mol Al mol Cu (most) discard (ii) mass of copper produced from copper(II)sulphate pentahydrate xg(Cu) = 0.0’37’5 mol CuSO4•5H2O X 3 mol Cu X 63.5 g = 2.4 g Cu reactant 3 mol CuSO4•5H2O mol Cu (least) The keeper, that is the limiting reactant, is: CuSO4•5H2O 4. What reactant is in excess? Al 5. How many moles of reactant are in excess? n(Al)reacted: limiting reactant X reactants’ mole ratio= n (excess reactant) 0.0’37’5molCuSO4•5H2O X 2 mol Al = 0.025 mol Al reacted 1 3mol CuSO4•5H2O n(Al)excess: 0.0926 mol(started)-0.025 mol(reacted) =0.068 mol in excess 4 dp 3 dp 3 dp and 2sf 6. Calculate the mass of reactant in excess? n(xs)= 0.0’67’6 mol X 27.0 g = 1.8 g Al that didn’t react mol 7. Find the mass of aluminum sulphate. Road Map: n(limiting reactant) n(product) xg (product) xg(Al2(SO4)3) = 0.0’37’5 mol CuSO4•5H2OX 2 moles Al2(SO4)3 X 342.3 g = 8.6 g 1 3 moles CuSO4•5H2O mole Al2(SO4)3 8. Find the mass of copper produced. xg(Cu) = 0.0’37’5 mol CuSO4•5H2O X 3 moles Cu X 63.5 g = 2.4 g limiting reactant 3 moles CuSO4•5H2O mol Cu