Laboratory Investigation: Enzyme Activity and Inhibitors
advertisement
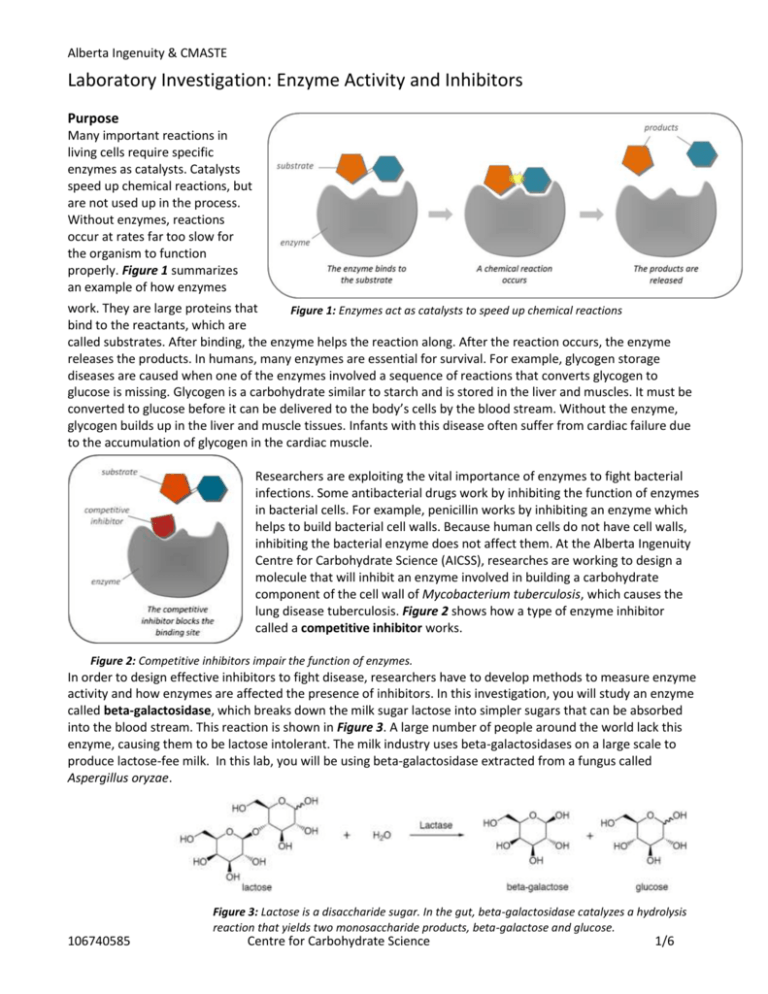
Alberta Ingenuity & CMASTE Laboratory Investigation: Enzyme Activity and Inhibitors Purpose Many important reactions in living cells require specific enzymes as catalysts. Catalysts speed up chemical reactions, but are not used up in the process. Without enzymes, reactions occur at rates far too slow for the organism to function properly. Figure 1 summarizes an example of how enzymes work. They are large proteins that Figure 1: Enzymes act as catalysts to speed up chemical reactions bind to the reactants, which are called substrates. After binding, the enzyme helps the reaction along. After the reaction occurs, the enzyme releases the products. In humans, many enzymes are essential for survival. For example, glycogen storage diseases are caused when one of the enzymes involved a sequence of reactions that converts glycogen to glucose is missing. Glycogen is a carbohydrate similar to starch and is stored in the liver and muscles. It must be converted to glucose before it can be delivered to the body’s cells by the blood stream. Without the enzyme, glycogen builds up in the liver and muscle tissues. Infants with this disease often suffer from cardiac failure due to the accumulation of glycogen in the cardiac muscle. Researchers are exploiting the vital importance of enzymes to fight bacterial infections. Some antibacterial drugs work by inhibiting the function of enzymes in bacterial cells. For example, penicillin works by inhibiting an enzyme which helps to build bacterial cell walls. Because human cells do not have cell walls, inhibiting the bacterial enzyme does not affect them. At the Alberta Ingenuity Centre for Carbohydrate Science (AICSS), researches are working to design a molecule that will inhibit an enzyme involved in building a carbohydrate component of the cell wall of Mycobacterium tuberculosis, which causes the lung disease tuberculosis. Figure 2 shows how a type of enzyme inhibitor called a competitive inhibitor works. Figure 2: Competitive inhibitors impair the function of enzymes. In order to design effective inhibitors to fight disease, researchers have to develop methods to measure enzyme activity and how enzymes are affected the presence of inhibitors. In this investigation, you will study an enzyme called beta-galactosidase, which breaks down the milk sugar lactose into simpler sugars that can be absorbed into the blood stream. This reaction is shown in Figure 3. A large number of people around the world lack this enzyme, causing them to be lactose intolerant. The milk industry uses beta-galactosidases on a large scale to produce lactose-fee milk. In this lab, you will be using beta-galactosidase extracted from a fungus called Aspergillus oryzae. Figure 3: Lactose is a disaccharide sugar. In the gut, beta-galactosidase catalyzes a hydrolysis reaction that yields two monosaccharide products, beta-galactose and glucose. 106740585 Centre for Carbohydrate Science 1/6 Alberta Ingenuity & CMASTE Problem 1. How does enzyme concentration affect enzyme activity? 2. How does the presence of a competitive inhibitor affect enzyme activity? Design In order to measure enzyme activity, an artificial substrate called p-nitrophenyl-beta-galactoside (pNP-Gal) will be used. This artificially synthesized compound consists of a beta-galactose linked to a ringed structure called pnitrophenol. The enzyme catalyzes the hydrolysis of pNP-Gal, breaking the bond that connects the betagalactose and the p-nitrophenol. This reaction occurs optimally at pH 5.0. The reaction will be allowed to run and then stopped by adding sodium carbonate solution increasing the pH to approximately 11. At this alkaline pH, the enzyme is deactivated and the p-nitrophenol product loses a proton to become an ion that reflects yellow light. The intensity of the yellow colour remaining after the reaction is stopped can be used as an indicator of enzyme activity. A very intense yellow indicates that relatively more pNP-Gal has been hydrolyzed. For the purposes of this investigation, enzyme activity will be defined as the ability of beta-galactosidase to catalyze the hydrolysis of pNP-Gal. Enzyme activity will be measured by analyzing a digital photo of the reaction mixtures using an image processing program. To test the first problem, enzyme solutions will be prepared with varying concentrations and a fixed amount of pNP-Gal will be added to samples of each solution. To test the second problem, the same process will be repeated but in the presence of lactose. Figure 4: pNP-Gal is used to measure the activity of beta-galactosidase. It is hydrolysed in a yield galactose and p-nitrophenol. At an alkaline pH, p-nitrophenol donates a proton to become an ion with an intense yellow colour. 106740585 Centre for Carbohydrate Science 2/6 SAFETY CONSIDERATIONS The following protective equipment should be worn: Safety glasses, gloves, apron Follow your teacher’s instructions concerning the disposal of chemicals Alberta Ingenuity & CMASTE Materials 10 ml test tubes, 6 Test tube rack Pasteur pipettes, 9 Bulb for Pasteur pipettes Immuno plate 10 ml graduated cylinder 0.1 M sodium acetate (NaAc) buffer, pH 5.0, 25 ml 5 mM p-nitrophenyl-beta-galactoside (pNP-Gal) in buffer, 5 ml 150 µg/ml A. Oryzae beta-galactosidase (enzyme) in buffer, 5 ml 0.5 M sodium carbonate (Na2CO3), 5 ml 125 mM lactose in buffer, 5 ml Digital camera (optional) Computer with ImageJ software (optional) Procedure: Part A: Preparing the enzyme solutions by repeated dilutions 1. Number your test tubes 1-6 and place them in the test tube rack in numeric order from left to right. 2. Use the graduated cylinder to measure 5 ml of the enzyme solution and transfer it to test tube 6. 3. Use the graduated cylinder to measure 0.5 ml of the enzyme solution in test tube 6 and transfer it to test tube 5. Add 4.5 ml of buffer to test tube 5. Mix the solution by covering and inverting several times. 4. Use the graduated cylinder to measure 0.5 ml of the enzyme solution in test tube 5 and transfer it to test tube 4. Add 4.5 ml of buffer to test tube 4. Mix the solution by covering and inverting several times. 5. Use the graduated cylinder to measure 0.5 ml of the enzyme solution in test tube 4 and transfer it to test tube 3. Add 4.5 ml of buffer to test tube 3. Mix the solution by covering and inverting several times. 6. Use the graduated cylinder to measure 0.5 ml of the enzyme solution in test tube 3 and transfer it to test tube 2. Add 4.5 ml of buffer to test tube. 7. Fill test tube 1 with 5.0 ml of buffer. Procedure: Part B: Testing enzyme activity 8. Place a clean Pasteur pipette into each test tube and into each solution listed in Table 1. Be careful to avoid cross-contamination. Table 1 shows the drops to be placed in each well of the immuno plate. Only use wells in outside rows to allow for a better digital photo later. Well # A1 A2 A3 A4 A5 A6 B1 B2 B3 B4 B5 B6 Enzyme 2 2 2 2 2 2 2 2 2 2 2 2 Solution Buffer Lactose solution pNP-Gal solution Na2CO3 solution 2 2 2 2 2 2 0 0 0 0 0 0 0 0 0 0 0 0 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Photo 1: A good way to label the wells is to place the immuno plate on a piece of paper and write the labels on the paper. Table 1: the numbers indicate how many drops to place in each well. The test tube numbers correspond with the well numbers. For example, add the enzyme solution from test tube 2 to both wells A2 and B2. 106740585 Centre for Carbohydrate Science 3/6 Alberta Ingenuity & CMASTE 9. Add the enzyme solutions to the immuno plate (as prescribed in Table 1). Next, add the buffer and lactose solutions. 10. Wait 2 minutes. 11. Add the pNP-Gal solution to the immuno plate. 12. Wait 30 seconds. 13. Add the Na2CO3 solution. Design/Procedure Questions: 1. Which test tube acts as the “negative control” for enzyme concentration? 2. Explain why two drops of buffer solution are added to the “A” wells. 3. For each of the two problem statements, given earlier in this investigation, identify the manipulated variable, responding variable, and four relevant controlled variables. 4. Explain how the “A” wells can be considered as a “control group” when testing the effect of lactose on enzyme activity. 5. Provide a reason for waiting in steps 10 and 12. Evidence/Analysis: Qualitative 1. In words and/or using a chart, table or diagram, describe the colour of each reaction mixture after the sodium carbonate solution has been added. Use words or the following symbols: (-) for no reaction and (+), (++), (+++), etc, for varying degrees of yellow. 2. Referring to your observations, describe: a. The effect of enzyme concentration on enzyme activity. b. The effect of the lactose on enzyme activity. Evidence/Analysis: Quantitative Enzyme activity will be measured quantitatively by determining the relative “darkness” (i.e. “value”) of each solution. A digital photo of the solutions will be analyzed using image processing software. Instructions for using a free, downloadable program called ImageJ are included, but you may wish to use a different program. ImageJ is available for free download at the following website: http://rsb.info.nih.gov/ij/ 3. Take a photo of your immuno plate in profile (so that you are looking at the wells side-by-side vertically, as shown in Figure 5). If possible, use the “macro” setting on your camera to get as clear an image as possible. Use diffuse light; it is important that all wells receive the same amount of illumination. 4. Upload the photo to your computer, and open it with ImageJ. To do this, right-click on the image file, select open-with, and then select ImageJ. Two windows will open: one with the ImageJ toolbar and the other with the image itself. (See Figure 5) 5. For each well in the photo, select a large uniform region. From the toolbar, Select Analyze, and then Measure. A Results window will open. Select and measure all 12 wells. The measurements will be added one at a time to the Results window. Figure 5: Use ImageJ to measure the relative enzyme activity for each well. 106740585 Centre for Carbohydrate Science 4/6 Alberta Ingenuity & CMASTE 6. The “Mean” values in the Results window give the average brightness of each measured region of the photo. Copy the Mean values under the heading of “Average Photo Brightness” in the data table like the one to the right. You may choose to use a spreadsheet program to do this. 7. Calculate the enzyme concentrations using information presented in the Materials and Procedure sections. Enter the values into the data table. 8. Calculate the relative enzyme activity for each “A” well by subtracting its average photo brightness from the average photo brightness of well A1. Do the same for the “B” wells, except subtract each value from the average photo brightness of well B1. Enter the values into the data table. 9. Plot two graphs on the same set of axes – one for the “A” data the one for the “B” data. On the horizontal axis, plot enzyme concentration, and on the vertical axis, plot enzyme activity. For each series of data points draw a curve of best fit. 10. Based on your quantitative analysis, describe: a. The effect of enzyme concentration on enzyme activity. b. The effect of the lactose on enzyme activity. Evaluation 11. Is the relationship between enzyme concentration and enzyme activity directly proportional, or is it better described by a different mathematical relationship? In words, provide an explanation for the general shape of the graphs of enzyme activity versus enzyme concentration plotted in this investigation. 12. Explain the effect lactose had on the ability of beta-galactosidase to hydrolyze pNP-Gal. Can lactose be considered to be a competitive inhibitor? 13. Sketch a graph predicting the results of the same experiment if it were conducted with a. A greater concentration of lactose. b. A lesser concentration of lactose. 14. Is it more appropriate to consider pNP-Gal as a competitive inhibitor, rather than lactose? Explain why pNP-Gal was considered to be the substrate rather that the inhibitor in this investigation. 15. People with diabetes suffer from high concentrations of glucose in the blood due to insufficient amounts of the hormone insulin. It is possible to measure glucose concentration using a glucose meter. Imagine that you are a researcher looking for new ways to decrease blood glucose concentration in individuals with diabetes. You wish to test a newly synthesized chemical which may inhibit an enzyme involved in converting glycogen into glucose. Describe an experiment that would test the effect of the inhibitor’s concentration on the enzyme’s activity. Include the following as part of your response: Problem Design (including identification of the manipulated variable, responding variable and controlled variables) Author: M. Haak 106740585 Centre for Carbohydrate Science 5/6 Alberta Ingenuity & CMASTE 106740585 Centre for Carbohydrate Science 6/6








