Maggie Lowe - McManus Lab
advertisement

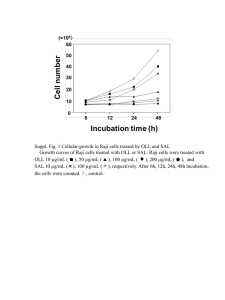
Maggie Lowe McManus Lab Fall Rotation 2008 Analysis of miR-Context Driven shRNA Expression in Immune Cells RNAi gene knockdown is a powerful tool to probe the function of the many uncharacterized proteins encoded by the human genome. Past RNAi systems have targeted an mRNA of interest with small interfering RNA delivered into the cell. Such systems have evolved into more stable and effective protocols utilizing lentiviral transduction to introduce a short hairpin RNA system into cellular DNA. These short hairpins, expressed through an RNA polymerase III promoter, must be converted into siRNA to exert their function. Unfortunately, the silencing produced by these systems is not always complete. More reliable and potent RNAi systems would benefit all RNAi technology, from targeting a single protein to screening entire genomes. Several methods are being developed to improve shRNA directed RNAi. By co-opting endogenous miRNA machinery to process the introduced shRNA, one might hope to have more efficient gene silencing. Flanking an shRNA with the pri-cursor arms found in endogenous miRNA might direct the shRNA into processing by Drosha and Dicer. Several laboratories have demonstrated that these miRNA context systems may dramatically increase the efficiency of gene knockdown.1,2 A separate area of interest lies in altering the promoter driving shRNA expression. Traditionally, polymerase III promoters such as U6 have been used. However, most miR-context approaches use powerful viral promoters, often the CMV promoter. This promoter has shown equal efficacy to U6 in knocking down gene expression in a shRNA system, despite being only moderately expressed in some cell lines.1 Taken together, a mir-context shRNA driven by the CMV promoter could be a more ideal system to produce potent and reliable RNAi. In the efforts to create a CMV promoter driven miR-context shRNA library, a test system was developed using GFP knockdown as a marker for efficacy. Past research in miR-context shRNA systems has mainly focused on the miR-30 endogenous pri-miRNA sequences. Therefore, antiGFP shRNA flanked by miR-30 sequences and driven by the CMV promoter was transduced via a lentivirus into GFP positive 293T cells; GFP knockdown was apparent via FACS analysis when transduction was successful. However, when this viral vector was introduced into GFP positive Raji cells, GFP knockdown did not occur in the infected cells. Understanding why these two cell types behave differently would allow for laboratory RNAi technology to move towards the development of miR-context shRNA libraries active across all cell types. In addition, this project could shed light on the regulation of siRNA and miRNA biology through deducing why this failure has occurred and what regulatory systems are implicated in these discrepancies. Aim 1: Investigate the cause of Raji cell miR-30-context shRNA-directed GFP knockdown failure. Changes in microRNA expression patterns during cell development, differentiation, and oncogenesis have pointed to extensive regulation of miRNA expression. Originally, transcriptional control was hypothesized to be the predominant regulatory mechanism for miRNA. However, discrepancies between the levels of primary transcript and mature miRNA have revealed that other cellular mechanisms downstream of transcription must also be active.3 Among these, inhibition of Drosha4,5 and Dicer6 processing, pre-miRNA A-to-I editing7, and enhancement of expression by Ago8 have all been shown to cause alterations in miRNA activity. By nature, these systems will be activated or inhibited in different cell types. Therefore, differential post-transcriptional regulation of a miR-30 context shRNA in Raji cells could be the cause of our system’s failure. Specifically, miR-30 flanking regions or the shRNA loop sequence may attract an inhibitor present in Raji cells but not in 293T cells that prevents miR-30 specific processing or miR-30 directed silencing. Such a factor has already been characterized in an embryonic stem cell line where the protein Lin28 specifically inhibited let-7 processing by Drosha4, 5, 9 or Dicer10 through interaction with the let-7 family loop sequence.4,9 Also, Raji cells may be deficient in an unknown protein, present in 293T cells, that is necessary for processing of the inserted shRNA or for silencing itself. Apart from endogenous miRNA regulationn, the lentiviral RNAi pathway itself may be responsible for the failure to silence GFP in Raji cells. Insufficient promoter strength could result in reduced shRNA production and thus insufficient GFP knockdown. The CMV promoter does have reduced potency in several immune cell types, including Raji cells. To better understand which of these possibilities play a role in the observed defects of GFP silencing in Raji cells, each of the above points will be investigated. First, the DNA sequence of the anti-GFP shRNA system will be analyzed, and both the flanking regions and loop sequence will be compared to wild-type miR-30 sequences. In particular, the loop region may be critical for RNAi function, as previous data has shown its importance in one instance of microRNA processing inhibition.5 Furthermore, published miRNA expression patterns for both Raji and 293T cells will be compared to determine similarities between the sequence of the shRNA used in previous experiments and wild-type miRNA typically expressed in each cell type.11 If the shRNA sequence closely resembles a member of the miR-30 family typically found in 293T cells but not Raji cells, then one might suspect that Raji cellular machinery might not process the miR-30 context shRNA as 293T cells would. The project then will branch in two separate directions. Anti-GFP shRNA with alternate flanking sequences will be generated, guided by what the sequence characterization of the previous shRNA systems reveals. In particular, a miR-142 context anti-GFP shRNA will be produced, since miR-142 is highly expressed in Raji cells, and such a system would be expected to work. Another set of experiments will focus on exchanging the CMV promoter for other promoters that might provide greater activity in Raji cells; such promoters might include Ubiquitin-c, eIF-1α, CAGGS, SV40, PGK, or the miR-155 promoter. These two branches of the project might interact; for instance, a miR-142 context shRNA might be combined with an alternate promoter, depending upon what the previous experiments have revealed. To assess whether miR-30 context shRNAs are correctly processed by endogenous Raji miRNA machinery, we will perform Northern blot analysis to study pri, pre, and mature anti-GFP shRNA expression in Raji cells. This will allow us to determine where the processing failure is occurring. The previous work involving mutating the flanking and loop sequences of the shRNA may be expanded dramatically in order to produce a functional shRNA. Multiple GFP positive cell lines will be produced to characterize which types of cells this knockdown failure will occur in. Finally, though likely too vast of an experiment for this rotation project, RNAi knockdown or cDNA overexpression studies could be performed in Raji cells to try to rescue the observed phenotype through inhibition or production of the factors that could differ between the Raji cells and 293T cells. In such an experiment, an shRNA library would be introduced into Raji cells that express miR-30 context anti-GFP shRNAs but cannot silence protein expression. These cells would be screened for GFP knockdown following the RNAi library transduction. Though some knockdown would be due to RNAi affecting GFP processing or GFP itself, some of hits might be from the silencing of an inhibitor of the miR-30 context shRNA. cDNA overexpression would produce the opposite effect. Providing the Raji cells with a protein present in the 293T cells but normally absent in Raji cells might allow for effective processing of the miR-30 context shRNA. The culmination of these efforts will result in a more thorough understanding of the biology of miR-30 context short hairpin RNA processing in Raji cells. References: 1. Chang, K., Elledge, S. J., & Hannon, G. J. (2006). Lessons from nature: MicroRNA-based shRNA libraries. Nature Methods, 3(9), 707-714. 2. Boden, D., Pusch, O., Silbermann, R., Lee, F., Tucker, L., & Ramratnam, B. (2004). Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Research, 32(3), 1154-1158. 3. Thomson, J. M., Newman, M., Parker, J. S., Morin-Kensicki, E. M., Wright, T., & Hammond, S. M. (2006). Extensive posttranscriptional regulation of microRNAs and its implications for cancer. Genes & Development, 20(16), 2202-2207. 4. Viswanathan, S. R., Daley, G. Q., & Gregory, R. I. (2008). Selective blockade of microRNA processing by Lin28. Science (New York, N.Y.), 320(5872), 97-100. 5. Newman, M. A., Thomson, J. M., & Hammond, S. M. (2008). Lin-28 interaction with the let-7 precursor loop mediates regulated microRNA processing. RNA (New York, N.Y.), 14(8), 1539-1549. 6. Obernosterer, G., Leuschner, P. J., Alenius, M., & Martinez, J. (2006). Post-transcriptional regulation of microRNA expression. RNA (New York, N.Y.), 12(7), 1161-1167. 7. Kawahara, Y., Zinshteyn, B., Chendrimada, T. P., Shiekhattar, R., & Nishikura, K. (2007). RNA editing of the microRNA151 precursor blocks cleavage by the dicer-TRBP complex. EMBO Reports, 8(8), 763-769. 8. Diederichs, S., & Haber, D. A. (2007). Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell, 131(6), 1097-1108. 9. Piskounova, E., Viswanathan, S. R., Janas, M., LaPierre, R. J., Daley, G. Q., Sliz, P., et al. (2008). Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. The Journal of Biological Chemistry, 283(31), 21310-21314. 10. Rybak, A., Fuchs, H., Smirnova, L., Brandt, C., Pohl, E. E., Nitsch, R., et al. (2008). A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature Cell Biology, 10(8), 987-993. 11. Landgraf, P., Rusu, M., Sheridan, R., Sewer, A., Iovino, N., Aravin, A., et al. (2007). A mammalian microRNA expression atlas based on small RNA library sequencing. Cell, 129(7), 1401-1414.