Chapter Three Notes
advertisement

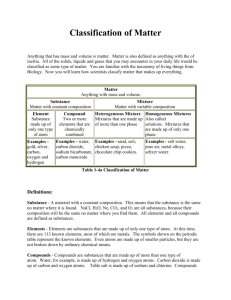
Chapter Three Notes- Elements, Mixtures, and Compounds Matter- is anything that has mass and takes up space. Substance- more properly called a pure substance. Matter Can it be separated by physical means? NO YES Pure Substance Can it be broken down by ordinary chemical means? Mixture Is the composition uniform? NO YES Element Oxygen Gold Compound Salt Baking Soda YES Homogeneous Salt water Alloy NO Heterogeneous Sand and water pizza I. Elements A. Examples: Cu, Pb, Al, and Zn B. Definition: Substance composed of only one type of atom C. Atoms- are the representative particle of elements. (This means the smallest particle that retains, or still has, the same properties as the whole). In other words, elements come in packages called “atoms”. 1. Subatomic particles a. proton (+), mass = 1 a.m.u. b. neutron (0) mass = 1 a.m.u. c. electron (-) mass = about 0 a.m.u. 2. Identity of Atom a. atomic number = number of protons 3. Neutral atomsa. the number of protons = the number of electrons = the atomic number b. the overall charge = 0 D. Types of Elements: 1. Monatomic a. exist in nature as single atoms in normal, standard conditions b. Examples: almost all elements 2. Homonuclear Diatomic a. exists in nature as two identical atoms bonded to form a molecule b. Examples: H2, O2, F2, Br2, I2, N2, Cl2. (Remember: HOFBrINCl) These must be memorized! 3. Polyatomic a. exists in nature as 3 or more atoms of the same element. Not many like this. b. Examples: Sulfur, Carbon 4. Isotopes a. atoms of the same element with different numbers of neutrons b. have the same number of protons, therefore, same chemical properties c. Examples: Carbon-12 and Carbon-13 (carbon 13 has an extra neutron) II. Compounds A. 4 Characteristics 1. They are two or more different elements, which are bonded, or chemically joined 2. The individual elements lose their properties. The compound has new properties. 3. The components are always in definite ratios. (This is the Law of Definite Proportions). For example, water is always H2O, or 2 hydrogens for every one oxygen. (2H:1O). 4. Separating the compound requires chemical means, i.e. usually a chemical reaction B. They always have a chemical formula. Examples: 1. Glucose C6H12O6 2. Methane CH4 3. Isopropyl alcohol C3H8O 4. Sodium chloride NaCl. There are many, many more compounds than elements! III. Mixtures: A. Definition: Physical combination of different substances B. Characteristics: 1. components retain (keep) their original composition and properties 2. components can have variable compositions. (For example, salt water may be 10% salt, and 90% water, or 50% salt and 50% water, etc.) 3. Mixtures can be separated by physical means a. distillation – separation based on differences in boiling points b. filtration – separation based on differences in particle size c. chromatography – separation based on differences in solubility of components C. Homogeneous Mixtures 1. Definition: physical combination of 2 or more substances that appears uniform throughout. Homogeneous mixtures are also known as “solutions”. 2. Made of two things: a. solute: substance being dissolved (less than 50% of the mixture) b. solvent: substance doing the dissolving (greater than 50% of the mixture) c. May be in any phase (a.k.a “state” of matter) 1.) solid dissolved in a liquid ex. Salt dissolved in water 2.) solid dissolved in another solid ex. Alloys such as bronze or 14K gold 3.) gas dissolved in another gas ex. O2 dissolved in N2 as in air D. Heterogeneous Mixtures 1. Definition: physical combination of 2 or more substances whose composition and properties vary throughout the sample. 2. Two types of heterogeneous mixtures: a. colloid (ex. Jello). Don’t settle over time, can’t be filtered out. b. suspensions (ex. Italian salad dressing) Will settle over time, can be filtered. Physical and Chemical Properties I. Physical Properties A. Definition: a property that can be observed without changing the identity of the substance B. Examples: mass conductivity ductility (can be shaped into wire) color boiling point freezing point density refractive index malleability (can be hammered flat) C. Two further categories 1. Extensive properties: a. definition: a physical property that depends on the amount of matter present b. examples: mass, volume, length c. not very useful in identifying unknown substances 2. Intensive properties: a. definition: physical property that does not depend on the amount of matter present. b. examples: density, boiling point, conductivity c. can be very useful in identifying unknown substances II. Chemical Properties A. Definition: a property of a substance undergoing a chemical reaction. B. Examples: Reaction with acid reaction with base Reaction with water reaction with air C. The property of NOT reacting is a chemical property as well! III. Physical Changes A. Definition: a change in which the same substance is present before and after the change. Physical changes are often reversible. B. Examples: 1. Shape changes: tearing, shredding, grinding, etc 2. Phase changes: There are 6 of these you need to know. Melting/Freezing, Sublimation/Deposition, and Boiling/Condensing 3. Dissolving IV. Chemical Changes A. Definition: a change of conditions that creates a new substance with new properties B. Examples: (There are many, many more) 1. rusting, as iron does when exposed to oxygen 2. cooking, as when a cake rises in the oven 3. digesting, as animals do with their food 4. burning, as in the combustion of fuels C. Recognizing chemical changes (Rules of Thumb) 1. formation of a precipitate ( a solid product) 2. change in color 3. production of bubbles (gas given off) 4. change in energy (temperature) a. endothermic- a reaction that absorbs energy from the surroundings b. exothermic- a reaction that gives off energy to the surrounding 5. change in smell/ new smell