Name
advertisement

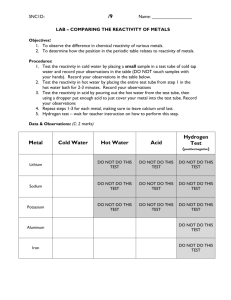
Mini-Lab: Periodic Table - Reactivity Patterns Purpose: To relate reactivity to the order of the Group 2 elements (alkaline earth metals) on the periodic table. To compare the similarities and differences of the reactivity of the four alkaline earth metal compounds. Procedure: 1. Fill 3 wells of MgCl2 half full. 2. Repeat step 1 for CaCl2, SrCl2, and BaCl2. Be careful you know which wells contain which chemicals. 3. Put 10 drops of K2CO3 in the wells with MgCl2, CaCl2, SrCl2, and BaCl2. Record your observations in the data table (No reaction, slight reaction, definite reaction). Look carefully!!! 4. Repeat step 3 with K2SO4 and K2CrO4. 5. Dispose waste in the waste tub. Clean your well plate with soap, water, and test brush. Lay it upside down on a paper towel to dry. Interpretations and Conclusions: Answer the following in complete sentences. 1. Which Group 2 element reacted the least? 2. Which Group 2 element reacted the most? 3. List the Group 2 elements in order from least reactive to most reactive. 4. How does your order of Group 2 elements compare to their order on the periodic table? 5. What is the relationship between degree of reactivity and the order of the alkaline earth metals (group 2) on the periodic table? 6. Based on your investigation of Group 2 elements, which metal in Group 1, other than francium, would you predict to be the most reactive? Least reactive? Mini-Lab: Periodic Table - Reactivity Patterns Purpose: To relate reactivity to the order of the Group 2 elements (alkaline earth metals) on the periodic table. To compare the similarities and differences of the reactivity of the four alkaline earth metal compounds. Procedure: 6. Fill 3 wells of MgCl2 half full. 7. Repeat step 1 for CaCl2, SrCl2, and BaCl2. Be careful you know which wells contain which chemicals. 8. Put 10 drops of K2CO3 in the wells with MgCl2, CaCl2, SrCl2, and BaCl2. Record your observations in the data table (No reaction, slight reaction, definite reaction). Look carefully!!! 9. Repeat step 3 with K2SO4 and K2CrO4. 10. Dispose waste in the waste tub. Clean your well plate with soap, water, and test brush. Lay it upside down on a paper towel to dry. Interpretations and Conclusions: Answer the following in complete sentences. 1. Which Group 2 element reacted the least? 2. Which Group 2 element reacted the most? 3. List the Group 2 elements in order from least reactive to most reactive. 4. How does your order of Group 2 elements compare to their order on the periodic table? 5. What is the relationship between degree of reactivity and the order of the alkaline earth metals (group 2) on the periodic table? 6. Based on your investigation of Group 2 elements, which metal in Group 1, other than francium, would you predict to be the most reactive? Least reactive? Data Table: K2CO3 MgCl2 CaCl2 SrCl2 BaCl2 K2SO4 K2CrO4