a cost-effectiveness analysis comparing single tablet
advertisement

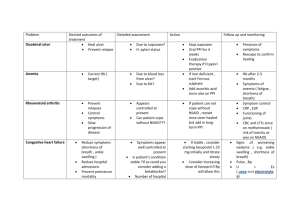
Abstractnummer: 2607 Gastroprotective strategies in chronic NSAID users: A costeffectiveness analysis comparing a single tablet formulation with individual component strategies N.L. de Groot1, P.D. Siersema1, N.J. de Wit2, B.M.R. Spiegel3, M.G.H. van Oijen1,3 1 Dept. Gastroenterology and Hepatology, University Medical Center Utrecht, Utrecht, the Netherlands 2 Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands 3 University of California Los Angeles/Veterans Affairs Center for Outcomes Research and Education (CORE), Los Angeles, CA, United States of America Patients with chronic arthritis are often treated with non-steroidal anti-inflammatory drugs (NSAIDs). However, NSAID-use is associated with a wide spectrum of gastrointestinal side effects, including dyspepsia, peptic ulcers and peptic ulcer bleeding. Co-therapy with proton pump inhibitors (PPI) is recommended for gastroprotection. However adherence to guidelines for prescribing PPI co-therapy and patient compliance to PPI is low. We evaluated the costeffectiveness of various therapeutic strategies in the prevention of gastrointestinal complications for chronic NSAID-users taking compliance to PPI into account. A cost-utility analysis was performed comparing 1) NSAID monotherapy, 2) NSAID and PPI co-therapy and 3) single tablet NSAID plus PPI formulation, by using a Markov model. The model was run for a hypothetical 60 year old patient with chronic arthritis requiring long term NSAID-therapy. Through series of 3-month Markov cycles, we followed the cohort over a 5- year horizon. Estimates on clinical outcomes, utilities and costs were derived from medical literature. Our primary outcome was incremental cost per quality adjusted life year (QALY) gained (ICER). Sensitivity analyses were performed to assess the effect of relative risk of GI complications, PPI compliance rates, and costs of PPIs and single tablet formulation. In our base case patient, NSAID and PPI co-therapy therapy was dominant (more effective and less costly) over NSAID monotherapy. The incremental cost-effectiveness ratio (ICER) of the single tablet formulation was €85,568 per QALY compared to NSAID and PPI coprescribed. In the sensitivity analysis, the single tablet formulation for the base case patient became “viable” (i.e. ICER < €20,000 per QALY gained) when the cost of PPI increased from €0.03 to €0.59 per tablet or when the costs of the single tablet formulation fell from €0.78 to €0.39 per tablet. PPI compliance did not influence the cost-effectiveness of the single tablet formulation in this base case patient. In high risk patients (patients with a 3x higher risk of GI-complications) the single tablet formulation was cost-effective (ICER €19,963). However, if PPI compliance increased beyond a threshold of 68%, NSAID and PPI coprescription became more cost-effective. Conclusion In chronic arthritis patients at average risk for gastrointestinal complications, NSAID and PPI co-therapy is the preferred treatment strategy. A single tablet formulation is only costeffective in high-risk patients with a low compliance to PPI therapy.