A Study on Composite Tissue Allotransplantation and
advertisement

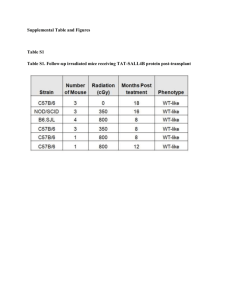
Title: Content of Bone Marrow in the Rat Bones and Planning of Flaps for Experimental Vascularized Bone Marrow Transplantation Authors: Fabio Quatra, MD, Oreste M. Romeo, MD, Dave Lowenberg, MD, Darrell Brooks, MD, Daniela Perri, MS, Lee Ann Baxter-Lowe, PhD, Michele R. Colonna, MD, Francesco Stagno d'Alcontres, MD, and Harry J. Buncke, MD The search for Tolerance in transplantation is highlighting the importance of Bone Marrow, which is highly immunogenic, may contribute to occurrence of rejection, may induce haematological chimerism (and possibly tolerance) or may be responsible for a lethal Graft-versus-Host Disease. The aim of this anatomical study was to evaluate the content of bone marrow in the various bones of the rat and to identify the corresponding flaps for their possible use in the study of vascularized bone marrow transplantation, comparing them to the rat hindlimb, one of the most popular models for the study of composite tissue transplantation. Materials and methods: Fifty rats (Sprague Dawley, Simonsen Lab. Inc.) were used, in a two-part study. In the first part, the content of bone marrow was evaluated by means of anatomical and analytical techniques, and the content of stem cells and mature lymphocytes estimated by flow cytometry. Briefly, bones were mechanically cleared of the soft tissues, fixed in buffered formalin for 48 hours, decalcified in a mixture of formic acid and calcium chelants for further 48 hours, and then were processed in different ways for bone marrow extraction and weigh Hematoxylin-Eosin stained sections, blindly performed with assessment of the ratio of viable bone marrow to fat degenerated marrow. Bone marrow was also extracted by freshly harvested bones, flushing the cavities with RPMI-1641 with the addition 4% Fetal Calf Serum and HEPES. After centrifugation for 10 minutes at 1,500 rpm, the pellet was washed twice in Hanff Balanced Salt Solution and red blood cells removed by a gradient of sedimentation (Lympholyte®, Cederlane Lab, Ontario). After centrifugation for 20 min at 1500 rpm, the pellet was resuspended, washed, filtered and cells counted with a fluorescent microscope after staining with acridin orange. About 500,000 cells were washed in Phosphate Buffered Solution and double labeled with an anti CD34 monoclonal antibody (FITC conjugated) as a marker for stem cells and an anti T-cell receptor antibody (PE conjugated) as a marker for mature T-lymphocytes (both antibodies from Cederlane Lab., Ontario) and counted on a BecktonDickinson FACSort flow cytometer. In the second part, we performed a surgical and anatomical study of the vascular pedicles and corresponding microvascular flaps, including vascular injection with latex and lead-oxide, x-rays and experimental in vivo transplantation (anaesthesia: Sodium Pentobarbital 40 mg/Kg i.p) when indicated. Results and discussion: Figures for volume and weight slightly differed between different bones, consistently with a different density and cellularity. As a general rule, cellularity showed a centripetal distribution, higher in axial skeleton and very little bone marrow could be found distally to the wrist or ankle with few nests of bone marrow sparse in the carpal bones and in the phalanxes. The highest cell-yield was from the tibia, followed by femur and pelvis, then sternum, scapula, humerus. Flow citometry showed a consistent amount of cells expressing the T-cell receptor. This cells, mainly mature T-lymphocytes, are often removed before bone marrow transplantation by means of magnetic beads or incubation with an anti-TcR antibody and Complement. Some cells expressing the TcR however, such as CD3+ CD25+ T-lymphocytes, are a possible candidate as “tolerogenic cell” responsible, or contributing, to the induction of immune tolerance. Selective removal of only a subpopulation of TcR+ cells, possibly responsible for the occurrence of Graft-versus-Host Disease, could be advisable. On the contrary, putative stem cells expressing the CD34 antigen accounted for less than 1% of the total content of leukocytes, with lower content in the sternum than in the long bones (Fig. 1). A dominant vascular pedicle is present in most bones, with few exceptions like the humerus (Fig.4). However, harvest of some flaps such as the pelvis or the acromioclavicular flap may be technically challenging. On the contrary the femur and tibia flaps rely on good-sized vessels originating from the common femoral vessels. We describe the microvascular transplant of a whole hemitorax, which intercostals system is vascularized both by the internal mammary vessels and the parietal branches of the thoracic aorta (see arteriogram in Fig. 2), and can be pedicled on the aorta and either caval or azygos vein. Possible advantages and disadvantages of this flaps are listed in Tab.1. Another new flap described is the cranial vault flap, in which the scalp is used as a vascular carrier, supplying the bone through a double row of paramedian perforators. The flap is pedicled on the common carotid artery and jugular vein. Its main advantage is to carry virtually no bone marrow. Conclusions: Only few flaps appear truly useful as experimental models. For isolated bone marrow transplantation, assuming that the bone itself is poorly immunogenic, the femur and tibia are the best combination of ease of dissection, size and length of the pedicle, and content of bone marrow. The hindlimb, including a modification developed by us with the removal of most of the bone marrow is probably still the best flap for the study of composite tissue transplantation. Table 1. Advantages and Disadvantages Advantages Long pedicle of large calibre Biggest flap available in the rat Possibly useful for mouse-to-rat transplantation of the Hemitorax Flap Disadvantages Tedious dissection Sacrifice of the donor Stealth syndrome in ratto-rat transplantation Fig.1: Putative stem cells expressing the CD34 antigen accounted for less than 1% of the total content of leukocytes, with lower content in the sternum than in the long bones Fig.2: A dominant vascular pedicle is present in most bones, with few exceptions like the humerus. From the left: humerus, tibia, femur, forearm. Fig.3: Arteriogram of the hemitorax flap