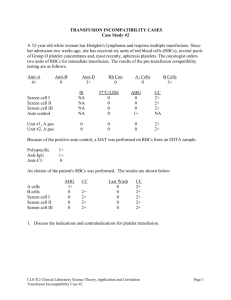
"On-call Manual" - Revision V (2008)
advertisement

The Ohio State University Medical Center Transfusion Service On-Call Manual GENERAL INFORMATION: The on-call resident pathologist will be responsible for the medical consultation of the Transfusion Service, Monday through Friday from 5:00pm until the day shift Transfusion Service Pathology Resident assumes responsibility in the morning at 8:30am and twentyfour hour coverage on weekends and holidays. Between 7:30 and 8:30am weekdays, the attending Transfusion Medicine physician is responsible for answering calls. Your primary responsibility will be directed consultation with the medical house staff concerning blood component usage, patient problems and apheresis procedures. Listed below are the typical hours of operation for each department within the Transfusion Service: 1. Main Laboratory- is open twenty-four hours, 365 days per year. 2. Reference/Prenatal Laboratory- is open Monday through Friday, excluding holidays from 8:00am through 4:30pm. 3. Apheresis Department- is open Monday through Friday, excluding holidays from 8:00am through 4:00pm. The Transfusion Service has additional staff available on an on-call basis: 1. Main/Reference/Prenatal Laboratory- a lead medical technologist is on call 365 days per year. Their function is to assist with serological problem resolution, after hours testing and staff coverage. a. They can be reached via either their home phone number or beeper 7317989. Home phone numbers are located in the Transfusion Service (2938467). 2. Apheresis- an apheresis nurse is on call for urgent after-hours and weekend procedures. They can be reached via beeper 730-7817. All other procedures should be scheduled during normal working hours. If for some reason the apheresis nurse on call cannot be reached by the beeper, home telephone numbers are located in the Transfusion Service and Apheresis Unit. Before leaving for the evening, it is recommended that the on-call resident pathologist checks with the Transfusion Service day resident or the Transfusion Service evening lead medical technologist or designated shift supervisor to be advised of any particular patient problems and/or blood inventory shortages. Likewise, the resident pathologist on-service for Transfusion Service should check the shift-to-shift communication log and with the morning shift lead medical technologist or designated shift supervisor, upon arriving in the morning. Also, check with the Apheresis Unit in case a late patient or emergency patient is anticipated. Page 1 The Ohio State University Medical Center Transfusion Service On-Call Manual TRANSFUSION SERVICE PANIC VALUES: The physician or person responsible for taking care of a patient must be notified immediately by laboratory personnel or the on-call resident pathologist of the following conditions: 1. Positive direct antiglobulin test on a cord blood and/or heelstick specimen. 2. Results of a transfusion reaction investigation, including gram stain and culture results (conveyed by the on-call resident pathologist). 3. Any clerical or technical error discovered after a blood component was issued for transfusion. 4. Delayed hemolytic transfusion reaction when the patient has been transfused within the last three months and developed a positive direct antiglobulin test or new alloantibody. 5. When blood components requested are not available because of inventory problems, serological problems and/or any other reasons. 6. Results of Kleihauer Betke test indicating a fetal maternal bleed. 7. An antibody titer of 1:32 or greater in a pregnant woman. 8. Results of Delta OD 450. 9. Results of ABO/Rh(D) on Lifeline of Ohio Organ Procurement specimens. 10. Results of subsequent compatibility testing on patients when blood was sent uncrossmatched. 11. Clerical or technical errors that involve patient reports or blood components. DISASTER PLAN: Modified Disaster Alert: This is a disaster of limited scope and only requires partial mobilization of Medical Center resources to be used for expansion of emergency services for the increased victims from one incident. Full Disaster Alert: This could involve the utilization of all community hospitals in the Columbus/Franklin County area. In order to ensure the appropriate utilization of the facilities resources while providing the best care to all our patients, the Medical Center has developed a two-tiered disaster plan. The tiered approach identifies a limited activation as well as a full activation of resources. The Transfusion Service responds to all disaster alerts (Code D). When the notification of a disaster is received, note the time of call, number of individuals involved and disaster status. The on-call Transfusion Service lead medical technologist is immediately notified. The on-call lead medical technologist will notify the on-call resident pathologist, determine increased staffing needs, coordinate blood and/or blood component inventory and transfusions and call-in needed personnel. The on-call resident pathologist must notify the attending pathologist and the Director of Clinical Laboratories. Page 2 The Ohio State University Medical Center Transfusion Service On-Call Manual During a disaster, there will be prioritizing of blood and blood components released from the Transfusion Service to patient care areas. Criteria for blood release at this time will be acutely hemodynamically unstable patients or patients with life threatening hemorrhage. Routine transfusions could be delayed until additional personnel become available in the Transfusion Service. a. Internal – an event in which only the Medical center is involved such as a fire, power outage or explosion. b. External – an event in which the Medical Center is not directly involved, but where the Emergency Department would receive a greater number of victims than can be treated under normal conditions. MAIN/REFERENCE/PRENATAL LABORATORY SPECIMEN REQUIREMENTS: The following are specimen requirements for serological testing: All of the following serological tests are performed routinely, twenty-four hours per day, 365 days per year. NOTE: All specimens and Transfusion Service requisitions must be signed (with physician’s pager number), dated and timed by the M.D., R.N. or Transfusion Service approved designee. 1. Type/Screen/Crossmatch- Two 5ml lavender (EDTA) tubes, unless the request is for greater than six units of packed red blood cells or the patient has atypical alloantibodies, three 5-ml lavender (EDTA) tubes. Transfusion Service specimen requisition. 2. Direct Antiglobulin Test- This request should also include a type and screen for evaluation of the indirect antiglobulin test (antibody screen). Two 5-ml lavendar (EDTA) tubes. General consult listing patient’s diagnosis, reason for consultation and current medications. 3. Cord Blood Workup- One lavender clearly marked “cord” blood. Transfusion Service specimen requisition. 4. Heelstick Workup- One brick heelstick bullet tube and Transfusion Service specimen requisition. 5. Rh Immune Globulin Evaluation- Two 5-ml lavender (EDTA) tubes and the Transfusion Service specimen requisition. the The following serological tests are performed routinely Monday through Friday form 8:00am through 4:30pm, excluding holidays: 1. Cold Agglutinin Titer- One cherry tube and a general consult. (Titers are done on Thursdays only.) Specimen is routed through the Central Processing Area. 2. Antibody Titer- Three 5-ml lavender (EDTA) tubes. Transfusion Service specimen requisition. Page 3 The Ohio State University Medical Center Transfusion Service On-Call Manual (*) 3. Kleihauer Betke- One lavender (EDTA) tube, a general consult listing the primary problem, gestational age and blood type if known. If the patient is in-house, it is recommended to have the patient type/screened. Kleihauer Betke’s are done on outpatients and specimen received from other facilities Monday through Friday, 8:00am through 3:30pm. 4. ABO Titer- One brick tube on the recipient and one yellow (ACD) tube on the donor. General consult. 5. Immunogenetic Markers- One yellow (ACD) tube on both the recipient and the donor. General consult. Delta OD 450- Amniotic fluid and Delta OD 450 consult. Cordocentesis/IUT- One lavender (EDTA) and 1-4 lavendar bullet tubes and amniotic fluid. Delta OD 450 consult. (*) 6. 7. (*)The Kleihauer Betke and the Delta OD 450 can be performed after hours on patients if clinically indicated. Refer to pages 37-38 for after-hour Kleihauer Betke/Delta OD 450 criteria. Page 4 The Ohio State University Medical Center Transfusion Service On-Call Manual HLA AND PLATELET SPECIMEN REQUIREMENTS: A. HLA/DR TYPING: 1. HLA/DR TYPING FOR ALL PATIENTS is done by the Clinical Histocompatibility Laboratory located in N-935 Doan Hall, (293-8554), 7:00am to 5:00pm. Below are guidelines for specimen collection, although you should contact Tissue Typing prior to having a specimen drawn. Diagnosis/Suspected Disorder Test(s) To Order Bone Marrow Malignancy 1. HLA-A, B, C Platelet Transfusion Refractory Neonatal Alloimmune Thrombocytopenia (NAIT) (Sent out test: Coordinate with Transfusion Service) 2. HLA-A, B 1. Platelet Phenotyping (mother and father). 2. Serum platelet Antibody (mother) Post-Transfusion Purpura (PTP) (Send out test: Coordinate with Transfusion Service) 3. Platelet antibody identification 1. Platelet antibody identification 2. Platelet phenotyping Sample Requirements WBC 500-1000 Drawn 40ml Sodium Heparin (green) WBC 1000-3000 Draw 30ml Sodium Heparin (green) WBC >3000 Draw 20ml Sodium Heparin (green) 30ml EDTA from mother and father 2. & 3. 5ml of serum from the mother 1. 5ml of serum as soon as possible after the onset of thrombocytopenia 2. EDTA sample (The volume guidelines are valid if a differential blood count reveals a lymphocyte value of greater than twenty percent. If there will be a greater than 24 hour delay in delivery, specimen(s) should be drawn in acd (yellow). Page 5 The Ohio State University Medical Center Transfusion Service On-Call Manual B. HLA/ ANTIBODY SCREEN HLA antibody screening is used to identify the presence of antibody(ies) directed against HLA antigens occurring on cells such as lymphocytes and platelets for platelet transfusion. The method used is flow cytometry. Unlike the HLA antigens on lymphocytes, which are intrinsically expressed, it is felt that HLA antigens on platelets are acquired due to the absorption of circulating, soluble HLA antigens. Sensitization to HLA antigens resulting from platelet transfusion support can contribute to immune clearance of transfused platelets and poor platelet transfusion response. This test is useful in identifying cases of platelet refractoriness due to an immune mechanism. For those patients exhibiting poor transfusion response as measured by the ten minute-one hour post transfusion increment, the HLA antibody screen confirm the presence of HLA incompatibility. Testing is done by the Clinical Histocompatibility Laboratory (293-8554), 7:00am to 5:00pm. Draw a 10ml brick top tube. The specimen should be drawn preferably at least 24 hrs post platelet transfusion. Transfusions are selected on the basis of HLA using the following definitions: 1. A match- identity of all HLA-A and –B antigens are identical. 2. BU match- two or three HLA antigens identical to the recipient with blanks in the balance of the phenotype. 3. BX match- two or three antigens identical, the remaining cross-reactive. 4. C match- three donor antigens match the recipient or are cross-reactive, while the fourth is mismatched. 5. D match- two or more donor antigens are non-identical to the recipient. C. PLATELET ANTIBODY SPECIMEN REQUIREMENTS Anti-platelet antibody and platelet-associated antibody are routinely done in Cellular Immunology. These tests must be scheduled through Cellular Immunology (293-8326), 7:30am to 2:00pm. Testing for heparin-induced thrombocytopenia is routinely done in the Coagulation Laboratory (688-6501), Monday through Friday, 7:30am to 2:00pm. The platelet antibody test is used for patients suspected of having allo- or autoimmune platelet antibodies (NAIT or ITP). It is also used as a rule-out for immunothrombocytopenia when other causes are suspected. Cases of suspected post transfusion purpura (PTP) are also investigated using the serum platelet antibody test. Platelet antibody, usually having anti-PLA1 specificity, can be detected in more than 90% of cases. Platelet phenotyping of the patient can be used additionally, to support a diagnosis of PTP. Page 6 The Ohio State University Medical Center Transfusion Service On-Call Manual D. NEUTROPHIL ANTIBODY ASSAY Both auto and allo-neutrophil antibody assays are sent to a Reference Laboratory through Central Processing Area. Central Processing Area (293-8375) is responsible for shipping the specimen(s). E. PLATELET CROSSMATCHING Platelet crossmatching should be attempted first (prior to HLA-matching) as a method to find compatible donors for patients refractory to platelet transfusion. Using a solidphase red cell adherence assay, the patient’s serum is tested against a panel of donor platelets. Even if a patient is broadly allo-immunized, compatible donors may still be found using this test, although the chance may be low. Platelet crossmatching involves mixing patient/recipient serum with donor platelets and assessing compatibility. This is believed to be more indicative of in vivo compatibility than is HLA-matching. In the absence of an existing HLA-antigen type of the patient, platelet crossmatching should be performed. Platelet-crossmatching specimen requirements: 1. Need 10ml of EDTA plasma from the patient. 2. Use the American Red Cross Platelet Testing Request form. NOTE: *If crossmatching with family member is requested, the American Red Cross needs an additional lavender (EDTA) tube from each family member to be crossmatched. F. PLATELET PHENOTYPING Neonatal alloimmune thrombocytopenia (NAIT) is the result of the destruction of fetal platelets by transplacentally acquired maternal antibody directed against paternal alloantigens. PLA1 incompatibility between the mother and the fetus accounts for 80-90% of NAIT cases. Platelet phenotyping of the mother and father often reveals that the mother is PLA1 negative and the father is PLA1positive, providing presumptive evidence for this diagnosis. Additionally, platelet antibody identification and a crossmatch of the maternal serum against the father’s platelets can be used to establish this diagnosis. Ideally, if the fetus/neonate should require platelet transfusion, the mother’s platelets, washed and suspended in saline, can be administered. This will require consultation with the medical director at the American Red Cross. If the mother is unable to donate, the American Red Cross will recruit a suitable platelet donor. Page 7 The Ohio State University Medical Center Transfusion Service On-Call Manual POLICY ON TRANSFUSION AND TURNAROUND TIME: All blood and/or blood component transfusion request(s) require the completion of the Transfusion Service Blood and/or Blood Component Order form. 1. Routine: The patient is on a regular nursing care unit, hemodynamically stable, but requires a blood transfusion as part of therapeutic regimen. 2. ASAP: The patient is bleeding, but hemodynamically stable, has a low hemoglobin or low platelet count. This also includes patients to be provided service on a priority basis (outpatient clinic and dialysis). 3. STAT: The patient is bleeding and hemodynamically unstable, or has the potential for sudden extensive blood loss or is having surgery within two hours. 4. TAT If a current specimen is available, the response time for a STAT order is 10-15 minutes. If no current specimen is available: Uncrossmatched group O Rh(D) negative red blood cells will be available within 5-10 minutes of receiving an order. Uncrossmatched type specific red blood cells will be available within 15-20 minutes of receiving the proper specimen and order. Complete type and screen and crossmatch will be done within 45-60 minutes of receipt of specimen. ASAP (Definition as above) Type and screen and crossmatch(es) will be completed within 2 hours of receiving the specimen. ROUTINE (Definition as above) Response time is 6-10 hours from time of specimen collection to administration of the blood component. ALLO-ANTIBODY IDENTIFICATION: 4-6 hours after receipt of the specimen. ALLO-ANTIBODY IDENTIFICATION WITH A POSITIVE DIRECT ANTIGLOBULIN TEST: routinely, 6-8 hours after receipt of the specimen. WARM AUTOABSORPTION: 12-16 hours after receipt of the specimen. ORDERS TO BE CLEARED BY THE ON-CALL RESIDENT PATHOLOGIST: NOTE: (Also refer to the current Transfusion Service Procedure Manual) The Transfusion Service technologists are required to intercept unusual orders and obtain the on-call resident pathologist approval before issuing the requested blood and/or blood components. The following is a list of unusual orders and recommendations: 1. Fresh Frozen Plasma (FFP) (see Transfusion Guidelines): Page 8 The Ohio State University Medical Center Transfusion Service On-Call Manual When the physician wants to transfuse more than four units of fresh frozen plasma/day to any patient or indications for transfusion are outside audit criteria and there is no medical indication, the Transfusion Service technologist will contact the on-call resident pathologist. The on-call resident pathologist should contact the ordering physician and determine if coagulation studies are abnormal or if there is other justification for transfusion. Hemorrhage, single/multiple coagulation factor deficiencies and DIC are some indications for fresh frozen plasma or pooled plasma solvent detergent treated transfusion. The Transfusion Service technologists normally attempts to obtain the laboratory results of the PT, INR, PTT, Fibrinogen, BUN, Creatinine, Platelet Count and Hgb, the patient’s diagnostic information and the name and beeper number of the ordering physician before calling the on-call resident pathologist. However, when the Transfusion Service technologist(s) are busy, the on-call resident pathologist may have to obtain all the information. 2. Cryoprecipitate AHF – (see Transfusion Guidelines): Cryoprecipitate is indicated for the treatment of hypofibrinogenemia, dysfibrinogenemia, congenital fibrinogen deficiency and consumptive coagulopathy. The Transfusion Service technologist will contact the on-call resident pathologist in ALL cases of orders for cryoprecipitate. Typically, 6 units of cryoprecipitate (cryo “pool”) are given to patients who have a dilutional coagulopathy due to massive transfusion. Uremic patients can also be given cryoprecipitate to aid in exposing the Von Willebrands factor for platelet adhesion, although this is not the first choice of therapy. Dialysis and DDAVP are more effective and should be used first. Estrogen therapy has been used in uremia with a more prolonged hemostatic effect. Single unit cryoprecipitate is used for fibrin surgical adhesive. Cryoprecipitate transfusions are indicated if the fibrinogen level is less than 100 mg/dL in bleeding patients. 3. Platelet Concentrates/Plateletpheresis (see Transfusion Guidelines): If the platelet count falls below 10,000 in a non-bleeding hematology or oncology patient or below 50,000 for surgical patients, platelet transfusion is indicated. Platelet counts of 5,000-10,000 (Lancet 338:1223, 1991) may be satisfactory in stable patients. This also allows for more judicious use of scarce/difficult-to-obtain platelet components. When more than 8 units of single, random donor platelet concentrates (2 platelet pools or 2 plateletpheresis) per day are requested for a patient, the Transfusion Service technologist will contact the on-call resident pathologist for approval. If the pre and 10 minute-up-to one hour post transfusion counts have not been done, the ordering physician should be encouraged to obtain pre and post transfusion platelet counts. If the post transfusion platelet count shows refractoriness to ABO matched random donor platelet apheresis on at least two occasions, the ordering physician should be encouraged to order crossmatched platelets (see page 39 and 40). 4. Other Situations Where The Transfusion Service May Request The On-Call Resident Pathologist Assistance: Massive Transfusion – Not Enough ABO/Rh(D) Identical Red Blood Cells: Page 9 The Ohio State University Medical Center Transfusion Service On-Call Manual When a massively transfused patient is using large amounts of red blood cell components (10 units in 24 hours), the Transfusion Service may not be able to continue providing ABO/Rh(D) identical red blood cells. Transfusing non-identical, but compatible ABO blood type red blood cells is not a major problem. Almost all of our red blood cells are collected in AS-1 (ADSOL) and have very little remaining plasma. A Transfusion Medicine physician’s approval is not required. However, having to give Rh(D) positive red blood cells to an Rh(D) negative patient requires the consent of the on-call resident pathologist. Rh(D) negative women of childbearing age (usually, but not exclusively, 45 years old) are never given Rh(D) positive red blood cells unless it is the only blood available and the medical condition (life-saving) warrants (see pages 33-35). 5. Not Enough Rh(D) Negative Platelet Concentrates and/or Plateletpheresis Available: Shortage of Rh(D) negative platelets occurs more frequently than shortage of Rh(D) negative red blood cells. When the patient in question is a hematology, oncology or bone marrow transplant patient requiring long-term platelet support, the Transfusion Service technologist will contact the on-call resident pathologist for the initial platelet transfusion of Rh(D) positive platelets. When the patient in question is a non-hematology or oncology patient, whose platelet transfusion support is not long-term, the on-call resident pathologist may approve transfusing Rh(D) positive platelets. If indicated, one vial of Rh Immune Globulin is enough to cover the transfusion of up to 30 Rh(D)-positive platelet concentrates or 6 plateletpheresis (see pages 33-35). 6. Massive Transfusion: When a patient is using a large number of red blood cells, but no fresh frozen plasma, cryoprecipitate and/or platelets, the Transfusion Service technologist will contact the on-call resident pathologist for assistance. The on-call resident pathologist should urge the patient’s physician to consider fresh frozen plasma and platelets, and to order appropriate coagulation studies. On the basis of the coagulation studies, the on-call resident pathologist may recommend fresh frozen plasma, cryoprecipitate and/or platelets to correct the dilutional coagulopathy. 7. Transfusion Reaction Complications: When a patient has an acute and/or delayed hemolytic transfusion reaction, the Transfusion Service technologist will contact the on-call resident pathologist. The on-call resident pathologist will notify the patient’s physician of the transfusion reaction. If the patient has signs and symptoms of an allergic, anaphylactic and/or febrile transfusion reaction, the Transfusion Service technologist will call the on-call resident pathologist with results of the initial transfusion reaction investigation. The on-call resident pathologist will advise the technologist if any additional testing is required (ABO, Rh(D), gram stain, culture, etc). The on-call resident pathologist should consult with the patient’s physician and advise medication and offer advise regarding clinical management (see pages 23-26). Page 10 The Ohio State University Medical Center Transfusion Service On-Call Manual 8. CMV Negative/Irradiated Blood Components: The first time a patient is requested to be given CMV negative and/or irradiated blood components, the on-call resident pathologist’s approval is required. Refer to guidelines on pages 30-31. 9. Blood Warmer: Refer to guidelines on page 30. WHEN CONSULTING WITH AN ORDERING PHYSICIAN AND A MODIFICATION IS MADE OF THE INITIAL ORDER, REMIND THE PHYSICIAN TO MODIFY THE ORDER. COMMUNICATE TO THE TRANSFUSION SERVICE THE DECISION REGARDING TRANSFUSION. ANTIBODY SCREEN (INDIRECT ANTIGLOBULIN TEST): Allo-antibodies are found in people who through pregnancy, previous transfusion, or injections have been exposed to foreign red blood cell antigens. Some people with no known immune stimulus may have unexpected antibodies, usually reacting at low temperature. Unexpected antibodies may be responsible for the following: 1. A discrepancy between ABO forward and reverse typing. 2. A positive antibody screening test. 3. An incompatible crossmatch. 4. A transfusion reaction. 5. Jaundice in a newborn. 6. A positive direct antiglobulin test. 7. A positive autocontrol. Antibodies stimulated by red cell antigens usually react in a predictable manner, depending on the specificity of the antigen. Some of the antigens stimulate the production of IgM and other IgG. The IgM antibodies react by agglutinating the red blood cells, while the IgG antibodies bind to the red blood cells, which then can be agglutinated by antiglobulin serum. Some antibodies (all IgM and some IgG) are able to activate complement. If the bound complement proceeds to completion of the sequence, the red blood cells will be lysed. If the sequence is not completed, the cell-bound components can be detected with polyspecific or monospecific anti-C3 antiglobulin serum. Routine antibody testing at The Ohio State University Medical Center is done by PEGantiglobulin method using immediate spin, 15 minute 37C incubation and the indirect antiglobulin test technique or Capture-R (solid phase adherence) technique. Capture-R is based on a modified solid phase technique using red cell adherence to detect IgG blood group allo-antibodies in the patient sera. Antibody screen red cells are bound and dried in a monolayer to the surface of polystyrene strip wells. The membrane antigens are used to capture red cell specific IgG antibodies. Following a brief incubation period, unbound residual immunoglobulins are rinsed from the wells and replaced with a suspension of antiIgG coated indicator red cells. Red cells for antibody screen are selected to have as many Page 11 The Ohio State University Medical Center Transfusion Service On-Call Manual antigens as possible. Antibodies reacting only at temperatures lower than 37C are generally considered to be clinically insignificant. If the antibody screen is negative by routine testing, only an immediate spin crossmatch is required. If a patient has a positive antibody screen, a complete routine panel is performed to identify the allo-antibody(ies). Antibody panels are composed of selected group O red blood cells from several donors who have been tested for as many of the common antigenic determinants as possible. These cells are different from each other and negative for several antigens. In addition to performing the antibody panel, the following additional tests are performed: 1. Direct antiglobulin test and autocontrol. 2. A test for the antigen(s) corresponding to the antibody(ies) suspected, unless the patient has been recently transfused. When ruling out alloantibodies, the following general principles apply: 1. Antibodies known to show dosage should be ruled out on the basis of homozygous cells. These include, but are not limited to, anti-Fya, Fyb, E, Jka, Jkb, M, C, S and s. Exceptions: Anti-E with anti-c, anti-S with anti-M, anti-C and anti-E with anti-D and anti-C with anti-e can be ruled out with heterozygous cells, because homozygous cells are rare. 2. Antibodies to low incidence antigens (Cw,Kpa, Jsa and Lua) need not be ruled out, rather rely on “crossmatch-compatible” blood. If a single and/or multiple antibody is suspected in a patient’s serum, at least 3 antigen positive panel cells must be shown to react. At least 3 antigen negative panel cells must be shown to be non-reactive. Page 12 The Ohio State University Medical Center Transfusion Service On-Call Manual Positive Antibody Screen Flow Chart Negative Panel Positive Panel Repeat Antibody Screen Identify Antibody(ies) Repeat ABSCR Positive Repeat ABSCR Negative Same phase and strength no additional testing required: Inconclusive Antibody No additional testing required Contamination Positive Crossmatch with Negative Antibody Screen Flow Chart 1. Obtain a new donor segment. 2. Check ABO of patient and donor. 3. Consider possibility of Anti-A1. Direct Antiglobulin Test on Donor Segment Negative Positive Repeat ABSCR and Xmatch Withdraw unit, Return to blood supplier All Negative Repeat ABSCR and panel Negative, Xmatch Positive No additional testing Frequency Antibody or Antibody Showing Dosage ESTIMATING BLOOD COMPATIBILITY: The frequency of blood group antigens can be used to calculate the probability of finding antigen-negative red blood cells. The calculation is relatively simple for a patient with a single blood group antibody. For example, if the serum of an individual contains anti-JK9 and the frequency of individuals lacking the JK9 antigen in the population is 23%, then approximately 23% or one out of every four ABO group specific red blood cells would be compatible. If more than one antibody is involved, it is necessary to multiply the frequencies of antigennegative donors. For example, if a Group A patient has anti-c, anti-JK9 and anti-Kell, the probability of finding compatible antigen-negative red blood cells: Page 13 The Ohio State University Medical Center Transfusion Service On-Call Manual 0.20 = probability of c negative 0.23 = probability of JK9 negative 0.90 = probability of K negative 0.40 = probability of A 0.54 = probability of O 0.11 = probability of B 0.04 = probability of AB To determine the probability, multiply the individual antigen frequencies and frequency of ABO compatibility. 0.20 X 0.23 X 0.90 X 0.40 = 0.01656 X 100 = 1.7% Frequency in A’s 0.20 X 0.23 X 0.90 X 0.54 = 0.022356 X 100 = 2.2% Frequency in O’s 0.20 X 0.23 X 0.90 X 0.94 (A’s + O’s) = 0.038916 X 100 = 3.89% Frequency in A’s and O’s If six red blood cells were needed for this patient, it would be necessary to screen 154 donor units of the compatible ABO type to find six compatible red cell. (see below) N = number of donor to be tested 3.89 = 6 100 N N = 600 3.89 = 154 units Once the degree of difficulty of the donor unit search is calculated, a rational approach to addressing the patient’s transfusion needs can be established and communicated to the medical housestaff. Page 14 The Ohio State University Medical Center Transfusion Service On-Call Manual RED CELL ANTIBODIES: Antibody % Compatible D C E c e Cw V G f K k Kp Kp Jsa Jsb Fya Fyb Jka Jkb M N S s U Lea Leb P/P1(Tja) Pla Lua Lub Xga Dia I i W = White B = Black 15 30 70 20 3 98 100W 82B 15 33 90 0.2 98 <0.1 99W 80B 0W<0.1B 33W 89B 20W 77B 25 25 22 28 45 11 0W 1B 78W 82B 22W 40B 0.1 20W 5B 92 1 36M 13F 99 0.1 99 M = Male F = Female Page 15 The Ohio State University Medical Center Transfusion Service On-Call Manual Associated withb Donor (%) Antibody Anti-A Anti-B Anti-A1 Anti-A,B Anti-D Anti-C Anti-Cw Anti-E Anti-c Anti-e Anti-M Anti-N Anti-S Anti-s Anti-U Anti-P1 Anti-P Anti-PP1pk Anti-Lua (Lutheran) Anti-Lub (Lutheran) Anti-K (Kell) Anti-k (Chilo) Anti-Kpa Anti-Kpb Anti-Jsa Anti-Jsb Anti-Lea (Lewisa) Anti-Leb (Lewisb) Anti-Fya (Duffya) Anti-Fyb (Duffyb) Anti-Jka (Kidda) Anti-Jkb (Kiddb) Anti-Xga (x-linked Antigen) Anti-Dia (Diegoa) Anti-Dib (Diegob) Anti-Yta (Cartwrighta) Anti-Ytb (Cartwrightb) Anti-Doa (Dombrocka) Anti-Dob (Dombrockb) Anti-Coa (Coltona) Anti-Cob (Coltonb) Anti-Sc1 (Scianna1) Anti-Sc2 (Scianna2) Anti-Ch (Chido) Anti-Rg (Rodgers) Anti-Kna (Knopsa) Anti-Knb (Knopsb) Anti-Yka (Yorka) Anti-SLa (McCoya) HDN HTR Yes Yes Yes Yes ? Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Few Few Rare ? Yes Yes Yes Yes Yes Yes No Rare No ? Rare ? No ? Mild Yes Yes Yes Yes Yes Yes Yes ? Yes ? Yes ? No Few No ? Yes Yes Yes Yes Yes Yes Yes Yes No report Yes Yes Yes Yes No Yes No Report ? Yes No Report Yes ? No Report No Report No Report No Yes No No No No No No No No No No Compatible White Black 56 69 85 76 53 52 45 49 15 8 30 68 99 100 70 98 20 1 2 2 22 30 28 26 45 69 11 3 0 <1 21 6 Extremely Rare Extremely Rare 92 0.15 91 98 0.2 Rare 97.7 100 Rare 0 100 80 0 1 78 77 28 45 34 22 17 77 23 9 28 57 M, 34 F,11 100 0 0.2 92 33 17 0.3 89 Very rare 99.7 - Data are from the American Association of Blood Banking Technical Manual (1985). HDN: Hemolytic Disease of the Newborn; HTR: Hemolytic Disease Transfusion Reaction Page 16 The Ohio State University Medical Center Transfusion Service On-Call Manual CROSSMATCH/COMPATIBILITY TEST: Routinely an immediate spin major crossmatch, testing of donor cells with the recipient’s serum, is performed on all patient specimens with a negative antibody screen. If the patient’s serum has a clinically significant antibody, a complete major crossmatch (immediate spin – AHG) is performed, which requires a 15-minute 37C incubation. Antibody Anti-D,C,E,c,e, Jka,Jkb,S,s,K,k Crossmatch Fya,Fyb, Donor red blood cells must be antigen negative. Complete major crossmatch. Anti-A1,P1,Lea,Leb Donor red blood cells do not have to be antigen negative. Rely on complete major crossmatch. Anti-M,N If antibody reactive at 37C and/or AHG, the donor red blood cells must be antigen negative. Otherwise, rely on complete major crossmatch. Anti-Jsa,Wra, Lua, Cw,Kpa,V Donor red blood cells do not have to be antigen negative. Rely on complete major crossmatch. The crossmatch will not: 1. Guarantee normal donor red cell survival. 2. Prevent recipient alloimmunization. 3. Detect all ABO grouping errors. 4. Detect Rh(D) typing errors. 5. Detect all unexpected red blood cell antibodies in the recipient serum. All red blood cell containing components (i.e., packed red blood cells, whole blood, granulocytes) must be crossmatched before being administered. Platelets usually contain less than .5ml of red blood cells and, therefore, do not require crossmatching. “Bloody” platelets should be returned to the supplier. Selection of Red Blood Cells for Crossmatch: 1. Select older red blood cells for patients with transfusion orders, and fresher red blood cells for surgical patients. 2. Infants should receive CPDA-1/CPD red blood cells, generally less than 14 days old and CMV negative. Adsol has been used for neonatal transfusion (Children’s Hospital). Page 17 The Ohio State University Medical Center Transfusion Service On-Call Manual Selection Of Blood Components When ABO Identical Donors Are Not Available ABO GROUP OF RECIPIENT ACCEPTABLE BLOOD GROUP OF DONORS Red Blood Cells O A B AB None A,O B,O AB, A, B or O ABO and Rh(D) must be compatible with the recipient’s plasma. Fresh Frozen Plasma O A B AB O, A, B, or AB A, AB B, AB None ABO should be compatible with the recipient’s red cells. Rh(D) typing need not be a consideration. Platelet Concentrate* O A B AB O, B, A, or AB A, B, AB, or O B, A, AB, or O AB, A, B Although all ABO groups are acceptable, ABO identical components are preferred. Platelet compatibility with recipient’s plasma is preferred. HLA match and platelet crossmatched are given regardless of ABO type. If more than 350 ml of incompatible plasma, the component should be plasma reduced. Cryoprecipitate O A B AB O, A, B, or AB O, A, B or AB O, A, B or AB O, A, B, or AB All ABO and Rh(D) groups are acceptable since the volume administered is small, unless volume exceeds 350 ml of ABO compatible plasma. COMPONENT RATIONAL NOTE: Type O packed red blood cells ADSOL (AS-1) are considered safe for transfusion for patients of any blood type. The concern for plasma incompatibility is unwarranted in view of the small amount of plasma remaining in an ADSOL (AS-1) pack red blood cell. *Platelet Concentrates/Pheresis: Every effort should be made to give ABO/Rh(D) identical platelets or at minimum ABO compatible platelets. Page 18 The Ohio State University Medical Center Transfusion Service On-Call Manual DIRECT ANTIGLOBULIN (COOMBS) TEST (DAT): The direct antiglobulin test (DAT) is used to detect the in vivo sensitization of red blood cells with IgG and/or complement. The DAT is useful for: 1. Diagnosis of hemolytic disease of the newborn. 2. Diagnosis of autoimmune hemolytic anemia and cold agglutinin disease. 3. Investigation of red blood cell sensitization caused by drugs. 4. Investigation of transfusion reactions. Positive Direct Antiglobulin Flow Chart Positive Polyspecific DAT Monospecific Testing DAT Positive IgG with DAT Positive C3 only or without C3 No More Testing Patient not transfused past 3 months Patient transfused within past 3 months DAT Positive 1st time this admission Positive DAT previously during this admission Eluate No Eluate Needed When interpreting a positive DAT, consideration must be given to the medication history, diagnosis, obstetrical history and transfusion history of the patient. 1. Polyspecific positive, monospecific negative: This situation is seen in patients with an increased reticulocyte count and in some cases due to the weak, synergetic effect of anti-IgG and anti-C3 in weak sensitization due to both IgG and C3. 2. Polyspecific positive, C3 only positive: a. Patients with Cold Hemagglutinin Disease. The antibody screen should show the presence of a cold autoagglutinin. b. 10-20% of Warm Autoimmune Hemolytic Anemias. c. Numerous medications cause in vivo complement binding on red cells (significant hemolysis seen with 3rd generation cephalosphorins). Page 19 The Ohio State University Medical Center Transfusion Service On-Call Manual d. C3 alone coated on the red cells in various neoplastic and collagen-vascular disease. 3. Polyspecific positive, monospecific positive: a. If a positive direct antiglobulin test is due to IgG, with or without a C3 component, an eluate must be performed. b. Both anti-IgG and anti-C3 are positive in 40-50% of cases of warm autoimmune hemolytic anemia. ELUTION: Red cell elution is the process of dissociating coated antibody from the membrane of the red blood cells. This makes the antibody available in a medium in which it can be tested for specificity. Interpretation of Eluate Results: 1. Eluate Nonreactivea. Drug induced (i.e. penicillin). b. Inadequate coating of antibody. 2. Eluate Reactive with all Cellsa. Drug induced (Aldomet, i.e., alpha-methyldopa). b. Warm autoantibody. 3. Eluate Reactive With only A and/or B Cellsa. Transfusion of ABO incompatible plasma (i.e. previous transfusion of platelets). b. Hemophiliacs (factor concentrate). 4. Eluate Reactive with Certain Cellsa. Antibody production (delayed transfusion reaction). b. Non-specific reactivity. c. IVIG (Anti-D and Anti-Kell have been reported). Drugs That Have Been Reported To Induce RBC Drug-Independent Antibodies (i.e., Autoantibodies) Page 20 The Ohio State University Medical Center Transfusion Service On-Call Manual Group 1a Methyldopa Levodopa Mefenamic acid Procainamide Catergenb Chaparralb Cyclofenilb Ibuprofenb a These b Group IIc Azapropazone Carbimazole Cefoxitin Chlorinated hydrocarbons Cianidanol Diclofenac Galfenine Latamoxef Nomifensine Phenacetin Streptomycin Teniposide Tolmetin drugs induce drug-independent antibodies only. More evidence is needed to prove that these drugs really can induce RBC autoantibodies c These drugs induce drug-independent antibodies together with antibodies reacting by different mechanisms. Associated With Antibodies Showing Characteristics Of More Than One Mechanisms a Drug Mechanisma a Chlorinated hydrocarbons AA + DA + IC Phenacerin AA + IC Streptomycin AA + DA Azapropazone AA + DA Teniposide AA + IC Galfenine AA + IC Latamoxef AA + IC Nomifensine AA + IC Cianidanol AA + DA + IC Diclofenac AA + IC Carbimazole AA + IC Tolmetin AA + IC Zomepirac AA + IC Cefotaxime AA + IC Ceftazidime AA + IC Cefotetan AA + DA and DA + IC Mechanisms are those described in the literature as (1) autoantibody (AA), (2) drugabsorption (DA), (3) “immune complex” (IC). Page 21 The Ohio State University Medical Center Transfusion Service On-Call Manual Drugs That Have Caused Immune Hemolytic Anemia and/or Positive Direct Antiglobulin Test (DAT) Acetaminophen Amephotericin B Ampicilin Antazoline Apronal Butizide Carbenicillin Carbimazole Cabromal Cefamandole Cefazolin Cefotetan Cefoxitin Ceftazidime Deftriaxone Cephalexin Cephaloridine Cephalothin Chlorinated hydrocarbons (insecticides) Chlorpropamide Chlorpromazine Cianidanol Cisplatin Cyclofenil Diglycoaldehyde Dipppyrone Crythromycin Fenoprofen Fluorouracil (5-FU) Floursemide Glafenine Hydralazine Hydrochlorothiazide 9-Hydroxy=methylEllipticinium Ibuprofen Insulin Isoniazid Latamoxef Levodopa Mefenamic acid Melphalan Methadone Methicillin Methotrexate Methyldopa Methysergide Nafcillin Nomifensine p-aminosalicylic acid Penicillin G Phenacetin Podophyllotoxin Probenecid Procainamide Pyramidon Quinidine Quinine Ranitidine Rifampicin Sodium Pentothal Streptomycin Sulphamides Teniposide Tetracycline Thiopental Tolbutamide Tolmetin Triamterene Trimellitic anhydride Zomepirac Drugs were included only when reasonable evidence was presented in the literature to support that they caused the immune reaction. There are many more reported, but the evidence that they caused a positive DAT or drug-induced hemolytic anemia is often minimal or totally lacking. Adapted from AABB Technical Manual, Principles of Transfusion Medicine (Rossi, et al), DAT Workshop. Page 22 The Ohio State University Medical Center Transfusion Service On-Call Manual WARM AUTOABSORPTION: In cases of warm autoimmune hemolytic anemia and warm autoagglutinins induced by drugs, such as Aldomet, not only may the direct antiglobulin test be strongly positive due to IgG or IgG/C3 but a warm autoagglutinin may be demonstrable in the serum. This antibody usually reacts with all donor and panel cells tested. In these cases, it is essential to know if there is an alloantibody being masked by the autoagglutinin. This can be accomplished by eluting some of the autoantibody off the patient’s red cells and then using these cells to absorb the autoagglutinin from the patient’s serum. Important consideration: 1. This procedure is performed with heterologous cells if the patient has been transfused in the past three months. The results of the autoabsorption studies should be interpreted with caution. Misleading negative results with the absorped serum may occur if the transfused cells possess the relevant antigen and have absorbed an alloantibody from the test serum. The American Red Cross can perform a heterologous absorption in problem/transfused cases. NOTE: Heterologous absorption can absorb out high-incidence antibodies. COLD AUTOAGGLUTININS: Cold autoagglutinins are present to a greater or lesser extent in all human sera. Under certain circumstances (i.e., monoclonal/polyclonal gammopathies, viral and/or pneumococcal infection), cold agglutinins may become extremely high titered. Most of the specificity of these antibodies is directed against antigens of the I system or related antigen systems. In the Transfusion Service, a cold agglutinin can cause all ABO and Rh(D) typing tests to be agglutinated with a positive reaction in the immediate spin phase of the antibody screen and/or crossmatches. The reaction is usually weaker at 37C. Most non-pathological cold agglutinins are primarily reactive below 32C and cause serological problems by binding complement. The presence or absence of clinically significant alloantibodies can be demonstrated by doing a regular pre-warm antibody screen. The pre-warm technique eliminates the carryover of the immediate spin agglutination into the indirect antiglobulin tests. If a pre-warm crossmatch is required for a resolution of a positive antibody due to cold agglutinins, any blood components given must be given through a blood warmer (see page 30). Most cold IgM antibodies will bind complement to red cells. This binding occurs best at 4C, but may also occur at room temperature. TRANSFUSION REACTIONS: A transfusion reaction is any unfavorable event occurring in a patient during or following transfusion of blood components that can be related to the transfusion. Since compatibility testing is performed for the detection of antibodies to red blood cell antigens, adverse effects of transfusion are most commonly caused by leukocytes, platelets, and plasma proteins. The most common causes of an acute hemolytic transfusion reaction are clerical errors and lack of proper patient identification. After completing the clerical check, DAT and hemolytic check, the Transfusion Service technologist will consult the on-call resident pathologist if more detailed serological work- Page 23 The Ohio State University Medical Center Transfusion Service On-Call Manual up is required or if the remaining blood and/or blood component needs to be gram stained and cultured. Acute Hemolytic Transfusion Reaction: Hemolysis of transfused red blood cells occurs infrequently but may cause a severe reaction accompanied by hemoglobinemia, hemoglobinuria, hypotension, DIC, acute renal failure and death. Initial symptoms are not diagnostic of hemolysis and often consist of fever, chills, flushing, a feeling of apprehension, chest or back pain, and nausea or vomiting. During anesthesia, the development of diffuse bleeding may be the only evidence of hemolytic reaction. Red blood cell destruction may be primarily intravascular, as seen in ABO incompatible red blood cell infusion or predominately extravascular as in Rh incompatibility. The Transfusion Service will perform a direct antiglobulin test, visual inspection for hemolysis, and a clerical check. In the event of a hemolytic transfusion reaction, the on-call resident pathologist should immediately call the on-call attending pathologist. If the reaction results in death, the FDA must be notified within 24 hours of ascertaining the transfusion was causal to the dealth. Recommend these tests to the patient’s physician, if clinically indicated: Test Specimen Times Serum Hemoglobin 1. Pre-transfusion 2. Immediate Post Serum Haptoglobin 1. Pre-transfusion 2. Immediate Post Serum Bilirubin 1. Immediate Post 2. 24-Hour Post Urine Hemoglobin 1. Immediate Post BUN/Creatinine 1. Immediate Post 2. Daily DIC Workup 1. Immediate Post Febrile Non-Hemolytic Reactions: Febrile non-hemolytic reactions, often preceded by chills, constitute the bulk of the transfusion reactions. These reactions are generally considered to be a result of cytotoxic or agglutinating antibodies in either the donor or recipient plasma directed against antigens present on lymphocyte, granulocyte or platelet membranes. While reactions are usually mild and result principally in recipient anxiety and discomfort, a hemolytic transfusion reaction must be ruled out. Pre-medication with antipyretics may modify reactions in some recipients. Leukocyte-reduced red blood cells and platelets help prevent these reactions. A febrile non-hemolytic reaction is a temperature rise of 1C or 2F or more occurring during transfusion or up to 2-4 hours following transfusion and without other Page 24 The Ohio State University Medical Center Transfusion Service On-Call Manual explanation. The fever may be caused by the infusion of bacterially contaminated blood. Psychrophilic gram negative bacteria (e.g. Yersinia) can cause severe reactions characterized by high fever. If indicated, specimens are sent to the Clinical Microbiology Laboratory for culture and sensitivity and a STAT gram stain. Between 3:00pm – 7:00am, the Critical Care Laboratory will perform and read the gram stain. Allergic Reactions: Allergic reactions following blood or plasma transfusion occur in about 1% of patients and are usually relatively mild. Most consist of local erythema, hives and itching, usually without a fever. An allergic reaction can easily be treated and/or prevented by administration of antihistamines; rarely, solucortef is required. Anaphylactic Transfusion Reactions: Most anaphylactic transfusion reactions have unknown etiology. Rarely, this form of transfusion reaction may be encountered in a patient who is IgA deficient and has made IgG antibodies. IV steroids and antihistamines are given. Some patients also require bronchodilators and blood pressure support. Delayed Hemolytic Transfusion Reaction: Delayed hemolytic transfusion reactions usually result in extravascular removal of transfused cells from the circulation days to weeks following transfusion. Rarely, abrupt intravascular hemolysis may occur with certain antibodies, such as anti-Jka and antiJkb. Red blood cells that were compatible at the time of transfusion may be destroyed following antibody formation (anamnestic response). The direct antiglobulin test is usually positive although the indirect antiglobulin test may be negative until all transfused red blood cells have been eliminated from the circulation. Nearly all delayed transfusion reactions, except for the very uncommon transfusion reactions, which produce intravascular hemolysis, tend to be asymptomatic and are only manifest by a mild, gradual anemia and transiently positive direct antiglobulin test. TRALI: Transfusion-related acute lung injury (TRALI) should be considered whenever a transfusion recipient experiences acute respiratory insufficiency, chills, fever, cyanosis, hypotension and/or when an x-ray finding is consistent with pulmonary edema, but without evidence of cardiac failure. The severity of the respiratory distress is disproportional to the volume of blood infused. Donor antibodies to HLA or neutrophil antigens may react with the recipient’s leukocytes, which cause permeability of the pulmonary microcirculation and fluid enters the alveolar spaces. If specific antibodies are absent, TRALI may result from complement activation to generate anaphylatoxins C3a and C5a; direct aggregation of granulocytes into a leukoemboli or transfusion of cytokines that have accumulated in stored blood. Treatment typically consists of O 2 therapy and ventilatory assistance and intravenous steroids. Patients recover adequate pulmonary function in 2-4 days. Circulatory Overload: Page 25 The Ohio State University Medical Center Transfusion Service On-Call Manual Rapid transfusion or transfusion in a fluid-overloaded patient may cause acute pulmonary edema due to volume overload. Infusion of 25% albumin, which shifts large volumes of interstitial fluid into the vascular space, may cause circulatory overload. Circulatory overload should be considered if dyspnea, cyanosis, orthopnea, severe headaches, hypertension or congestive heart failure occur during or following transfusion. Graft versus Host Disease: T lymphocytes present in cellular blood components may cause transfusion-associated graft-vs-disease (GVHD), which results in some or all of the following findings: fever, dermatitis or erythroderma, hepatitis, enterocolitis, pancytopenia, and immunodeficiency. At present, there is no effective treatment for transfusion-associated GVHD. Gamma irradiation (minimum 1500 rads) of cellular blood components prevent transfusion-associated GVHD. Citrate Toxicity: When larger volumes of fresh frozen plasma, whole blood or platelet concentrates are transfused with rates exceeding 100 ml/minute, plasma citrate levels may rise and hypocalcemia may occur. Unless a patient has a predisposing condition that hinders citrate metabolism, hypocalcemia due merely to citrate overload requires no treatment other than slowing or discontinuing the transfusion. If a patient remains symptomatic, the patient may require careful intravenous infusion of 10% calcium gluconate. Calcium should not be administered through the access line for transfusion as the blood may clot. Cytokines: Leukocyte reduction has been used with considerable success to prevent febrile nonhemolytic transfusion reactions, but in many cases even near complete removal of the leukocytes is ineffective. It is known that cytokines are generated during storage, even at 1-6C but to a much greater extent at 20-24C. Cytokine levels rise in direct proportion to the number of leukocytes. The variety of cytokines produce a combined effect including fever, hypotension, mobilization of neutrophils from the bone marrow, activation of endothelial cells to express adhesion molecules and procoagulant activity, activation of T and B lymphocytes and priming of neutrophils. ESTIMATED RISKS OF TRANSFUSION: 1. Hepatitis B transmission per screened unit – 1:600,000 2. Hepatitis C transmission per screened unit – 1:1,200,000 3. HIV transmission per screened unit – 1:2,000,000 4. HTLV I/II transmission per screened unit – 1:641,000 HTLV infection – 4% lifetime risk of associated disease (adult T-cell leukemia, tropical spastic paraparesis). 5. Acute hemolytic transfusion reaction – 1:25,000 Page 26 The Ohio State University Medical Center Transfusion Service On-Call Manual 6. Non-hemolytic transfusion reaction – 1:200 7. Chills – 1:200 8. Fever – 1:200 9. Formation of allo-antibodies – 1:100 10. Non-Enveloped Viruses (Hepatitis A, Parvovirus B19): the risk of transmission is not determined. Parvovirus B19 can cause bone marrow hypoplasia. 11. Other risks – a. Circulation overload b. Bacterial contamination c. Iron overload 12. Other diseases: Yersinia, Malaria, Babesiosis, Chagas – 1:1,000,000 each. Overall: risk that blood recipient will have a serious or fatal transfusion transmitted disease – 1:20,000 RISK OF MASSIVE TRANSFUSION: 1. Hypothermia (RBC massive transfusion) 2. Air emboli if blood component transfused under pressure 3. Citrate toxicity (predominantly with FFP administration) 4. Metabolic alkalosis 5. Hypokalemia 6. Coagulation defect (predominantly dilutional coagulopathies) Page 27 The Ohio State University Medical Center Transfusion Service On-Call Manual TRANSFUSION REACTIONS Symptoms* Lab Findings* Treatment Hives None Antihistamines (Benadryl 50 mg p.o. or i.v.) Anaphylactic Laryngeal edema Bronchoconstriction Rarely, IgA deficiency or none Corticosteroids Febrile Non Hemolytic Fever, chills, shortness of breathe, tachycardia, flushing Usually none. Sometimes leukoagglutinin or lymphocytotoxic antibodies Antipyretics (Tylenol) Antipyretics leuko reduced Fever, chills, shock, acute renal failure Clerical errors, hemolysis, positive direct antiglobulin test. Elevated serum and urine bilirubin Fluids, diuretics (Lasix). Monitor renal output Careful identification of blood specimens and blood components Fever, chills, jaundice, dropping H/H. Sometimes no symptoms Positive direct antiglobulin test, and antibody screen (Hemolysis rare). Increased bilirubin. Monitor renal function Rarely can be prevented Shortness of breath, cough, pulmonary edema Chest x-ray Slow or stop infusion, patient sitting position, diuretics Use packed red blood cells. Avoid unnecessary transfusion of plasma Shortness of breath, pulmonary edema without cardiac failure Neutrophil or HLA antibodies Reaction Type Allergic Acute Hemolytic Delayed Hemolytic Circulatory Overload TRALI *Any Prevention Give antihistamine Support patient. Antihistamines Avoid unnecessary transfusion. Report donor to blood supplier one of any combination BLOOD ADMINISTRATION: 1. Recommended Needle Gauge to Maintain Red Blood Cell Integrity: Needles or catheters used for blood transfusion should be large enough to allow appropriate flow rates. An 18-gauge needle provides good flow rates for cellular components without excessive discomfort to the patient. A. Red blood cells/whole blood: 22-gauge or larger. B. Other blood components: 22-gauge or larger. Page 28 The Ohio State University Medical Center Transfusion Service On-Call Manual 2. IV Solution Compatibility: A. Normal saline (0.9 NaCl) is the only acceptable fluid. DO NOT USE: 5% dextrose/water (D5/W) – water causes the red blood cells to swell and rupture, hemolysis. Lactated Ringer’s (LR) – calcium in the solution counteracts the anticoagulant in the blood component permitting small clot formation. 3. Foot IV/Access: A foot IV should be used only as the last resort, when all other forms of access have been attempted. The on-call resident pathologist may be consulted by clinical colleagues regarding the need for transfusion using a foot IV. Note: This is because there is a greater risk for venous stasis and phlebitis in a leg vein. 4. A current type and screen (drawn within 3 days of the last RBC transfusion) is needed for transfusion of all blood and/or blood components, except for preadmission testing 2-3 weeks before when the patient has not been transfused or pregnant in the previous three months. Exception: Plasma and platetets and cryoprecipitate need only type and screen during that admission. AUTOLOGOUS/DIRECTED DONATIONS: Request for autologous and directed donations are handled through the American Red Cross, Special Needs Coordinator (614/251-1450), Monday through Friday, 8:00am through 5:00pm. Processing of each donation requires a minimum of 3-4 days (most often 5 days). All infectious disease testing is performed on each autologous and directed donation collected at the American Red Cross. The Transfusion Service has available prescription forms for autologous and directed donations. It is best to refer questions concerning autologous and directed donations to a lead medical technologist. Other Alternatives to Allogeneic Transfusion: 1. Intraoperative blood salvage. 2. Hemodilution. 3. Post operative blood salvage. 4. Pharmacological alternatives (e.g., vitamin K, Erythropoietin, iron). 5. Recombinant Growth Factor (e.g., erythropoietin, granulocyte-macrophage colonystimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF). 6. Fibrinolytic Inhibitors. 7. DDAVP. Page 29 The Ohio State University Medical Center Transfusion Service On-Call Manual BLOOD WARMER: The Transfusion Service has three blood warmers that are used in the following situations: 1. Patients with cold autoagglutinins requiring pre-warm techniques for resolution of serological problems. 2. Neonatal exchange transfusion. 3. Patient requiring massive transfusion to prevent hypothermia. 4. OB/GYN procedures (see below). The blood warmers are used for treating hypothermia and OB/GYN procedures (amnioinfusion to supplement amniotic fluid or to cleanse meconium) only if available. SICU and Labor and Delivery have their own blood warmers. Approval of the on-call resident pathologist is required for the use of a blood warmer when not initiated by the Transfusion Service. Hypothermia: Rapid infusion of larger volumes of cold blood can lower the temperature of the sinoatrial node to below 30C, at which point, ventricular arrhythmia can occur. Hypothermia can increase the toxicity of hypocalcemia or hyperkalemia and can result in serious lifethreatening ventricular arrhythmias or poor left ventricular performance. Treatment consists of using warming blankets and lights, warmed intravenous solutions and the use of a blood warmer. POLICY ON CMV NEGATIVE AND/OR IRRADIATED BLOOD COMPONENTS: Cytomegalovirus (CMV) can be a severe infectious complication in certain patients who are sero-negative and severely immunocompromised. CMV transmission can be prevented by leukocyte-reduced blood components or CMV sero-negative. However, only about 40% of the blood donors in Central Ohio are CMV sero-negative, limiting the supply of CMV sero-negative blood components. Therefore, CMV sero-negative blood components must be restricted to certain patient populations. Leukocyte-reduced blood can be used for these patients instead. Irradiated blood components are indicated to prevent graft-versus-host disease in the following situations: 1. BMT transplant. 2. Neonates and children immunodeficiency status. with severe or known congenital or acquired 3. IUT (intrauterine transfusion). 4. Recipients of cellular blood components from blood relatives (directed donation). 5. Patients with Hodgkin’s disease, lymphoma, AML (other malignancies less likely). 6. Neonates of weight less than 1500 grams. 7. HLA matched plateletpheresis components. Page 30 The Ohio State University Medical Center Transfusion Service On-Call Manual CMV sero-negative blood components (red blood cells and platelets) are reserved for the following patients: 1. Bone Marrow Transplant (BMT): a. All allogeneic (related or unrelated donor) recipients who are CMV negative. b. CMV sero-negative patients with acute bulemia who potentially are BMT candidate. 2. Heart Transplant: a. CMV sero-negative recipients and candidates. 3. CMV sero-negative (or unknown) pregnant women. 4. All neonatal infants and all fetuses. Other patients including liver, kidney, and pancreas transplant patients, will receive CMV untested leukocyte-reduced blood components. HEMOTHERAPY PATIENTS: FOR ALLOGENEIC BONE MARROW TRANSPLANT (BMT) For allogeneic BMT, the patient will receive ABO identical and/or compatible blood components, CMV negative (if the patient is CMV sero-negative), irradiated and leukocytedepleted. Exception: fresh frozen plasma and cryoprecipitate only need to be irradiated. ABO and/or Rh(D) incompatibility between bone marrow transplant donor and recipient does not adversely affect patient survival or development of graft-versus-host disease in BMT patients. However, ABO and/or Rh(D) of the blood components transfused to these patients must be carefully selected for both pre-BMT and post-BMT periods. 1. Rh(D) Incompatibility: When the recipient is Rh(D) positive and the BMT donor is Rh(D) negative, all packed red blood cells to be transfused starting from pre-operative regimen should be Rh(D) negative. During the post-operative period, when the recipient red blood cells are still demonstrable in the recipient’s circulation, Rh(D) positive plateletpheresis may be given without resident pathologist approval. However, once it is established that the recipient type red blood cells are no longer detectable in the recipient’s circulation, all blood components including plateletpheresis should be Rh(D) negative. The Reference Laboratory will be responsible for updating the patient problem folder, computer system and Transfusion Service requisition(s) on the basis of current serological testing. When the recipient is Rh(D) negative and the BMT donor is Rh(D) positive, the recipient should continue to receive Rh(D) negative packed red blood cells and plateletpheresis. After the Reference Laboratory has determined that the recipient has actually received the bone marrow transplant from a Rh(D) positive donor, the Reference Laboratory will update the patient problem folder, Transfusion Service requisition(s), and the computer system that the patient can receive Rh(D) positive blood components. Page 31 The Ohio State University Medical Center Transfusion Service On-Call Manual 2. ABO Incompatibility: When there is major or minor ABO incompatibility between the donor and recipient, the Reference Laboratory will make notations in the patient problem folder, Transfusion Service requisition(s) and computer system according to the following table: ABO INCOMPATIBILITY ABO OF BLOOD COMPONENTS PACKED FFP PLATELETS RED CELLS 1st Choice 2nd Choice O A, AB A,AB B,O O B, AB B,AB A,O O AB AB A,B,O DONOR ABO RECIPIENT ABO O O O A B AB A A A B O AB O O A AB A, AB AB AB A,AB AB A,B B A,B B B B A O AB O O B AB B, AB AB AB B,AB AB A,B A A,B AB AB AB A B O A B O AB AB AB AB AB AB AB AB AB 3. Post-Engraftment: The Reference Laboratory continues to receive specimens from BMT recipients after discharge. They will continue to monitor the following on post-transplant patients: Disappearance of circulating ABO antibody to donor type red blood cells and ABO titers if indicated. Appearance and approximate percentage of donor type red blood cells. Direct antiglobulin test. Transfusion history. 4. Blood Type Change: When a specimen is received more than three months from the last blood component transfused and the Reference Laboratory determines: All circulating red blood cells are of donor ABO type. Page 32 The Ohio State University Medical Center Transfusion Service On-Call Manual Recipient’s original ABO antibody directed against donor type red blood cells is no longer demonstrable. The patient has not been transfused in past three months. The direct antiglobulin test is negative. If all of the above criteria are met, the Reference Laboratory will send a general consult to the Bone Marrow Transplant Unit, and change the patient’s blood type in the computer system, patient problem folder and Transfusion Service requisition(s). From this point forward, the recipient will receive donor type red blood cells and plasma components. THERAPEUTIC PHLEBOTOMY: A request for a routine therapeutic phlebotomy could be arranged to be done by the Apheresis Department, during hours of normal operation. Emergency therapeutic phlebotomy is performed by members of the clinical unit, in which the patient is located. For emergent therapeutic phlebotomy, a kit is available from the Transfusion Service. NOTE: We do not perform re-infusion. Emergency phlebotomy is performed in some severe cases of pulmonary edema or polycythemia rubra vera. POLICY ON RH(D) NEGATIVE WOMEN OF CHILDBEARING AGE: Whenever a request for a type and screen is received on an Rh(D) negative women of childbearing age (usually but not exclusively 45 years old), it is the responsibility of the Transfusion Service to determine if the patient could be pregnant. If the patient is pregnant, appropriate measures should be taken to ensure Rh(D) immune globulin is administered when indicated. CORD BLOOD WORKUP: Cord blood samples are routinely collected at delivery and sent to the Transfusion Service. Cord blood testing is indicated in the following situations: 1. The mother is a Rh(D) immune globulin candidate (i.e., Rh(D) negative). 2. The mother has an antibody capable of causing hemolytic disease of the newborn. 3. To determine if ABO incompatibility exists in jaundiced infants (upon physician request). Page 33 The Ohio State University Medical Center Transfusion Service On-Call Manual Special considerations when testing cord blood: 1. Previous intrauterine transfusions. 2. Blocked Rh(D). If the direct antiglobulin test is strongly positive and the mother has anti-D, the cell’s antigen sites may be so completely coated with maternal antibody that the Rh(D) appears negative with any anti-D reagent. If this occurs, an eluate must be performed. The presence of anti-D in the eluate confirms that the red cells are Rh(D) positive. 3. If the direct antiglobulin test is positive, the Rh(D) weak status on Rh(D) negative infants cannot be determined. This can be resolved with chloroquine treatment of the cord blood cells to remove attached antibody. 4. Family paternal/maternal antigen-antibody incompatibility. RH(D) IMMUNE GLOBULIN: Rh(D) Immune Globulin is prepared from a pool of human plasma containing high tittered anti-D. The IM preparations are given as an IM injection and should never be administered IV. An IV preparation of Rh(D) Immune Globulin (WINRhO) is licensed and available. Although it can be used for Rh(D) negative mothers, it is most often used for ITP. Each vial of Rh(D) Immune Globulin (IM) contains 300 gm of anti-globulin, which has been documented to cover 30 ml of whole blood or 15 ml of packed red blood cells in the circulation. Rh(D) Immune Globulin acts to suppress the immune response of Rh(D) negative individuals exposed to the D antigen. There is no evidence that Rh(D) Immune Globulin will benefit a patient who is actively producing anti-D. The primary use of Rh(D) Immune Globulin is prevention of immunization in female of childbearing age, thus preventing future hemolytic disease of the newborn. Rh(D) Immune Globulin should be given less than 72 hours post-delivery, abortion, miscarriage, amniocentesis; although if 72 hours has elapsed, the Rh(D) Immune Globulin should still be given. No more than five vials of Rh(D) Immune Globulin per buttock at one time. Rh(D) Immune Globulin can be given over a span of 72 hours after the event. Indications: 1. Delivery of a Rh(D) or Rh(D) weak positive infant. 0.3% of deliveries have a 30 ml bleed. 1.0% have a 6 ml bleed. 2. Abortion or miscarriage (Fetal Blood Volume = 85 ml/kg). 3. Post amniocentesis. 4. Antepartum at 28 weeks. 5. Transplacental fetal-maternal hemorrhage, threatened abortion, external cephalic version, antepartum bleeding, maternal abdominal hemorrhage, or maternal trauma. Page 34 The Ohio State University Medical Center Transfusion Service On-Call Manual 6. Transfusion of Rh(D) positive blood and/or blood components to a Rh(D) negative individual. Random, single donor platelet concentrates generally contain <0.5 ml of red blood cells; therefore, one vial of Rh(D) Immune Globulin will suppress 30 or more Rh(D) positive random, single donor platelet concentrates or 6 or more plateletpheresis. Packed Red Blood Cells (RBC) Whole Blood (WB) Calculations: (RBC volume X .65 (ADSOL))/15 = number of vials (WB volume X .50)/15 = number of vials 7. Situations with greater than a 30 ml fetal maternal bleed: Random 0.3% Neonatal anemia: 26% Perinatal death: 11% Abrupto placenta: 7% Manual removal of placenta: Third trimester trauma: Third trimester amnio: Contraindications: 1. The mother has received Rh(D) Immune Globulin within 72 hours of the delivery and her fetal blood screening test on the post delivery specimen is negative. If the fetal blood screening test is positive, a stain for fetal hemoglobin (Kleihauer-Betke) will need to be done to determine the fetal bleed and the number of vials of Rh(D) Immune Globulin indicated. 2. The mother had an amniocentesis or an external cephalic version and received one vial of Rh(D) Immune Globulin within the past 21 days and there is a negative fetal screen. No additional Rh(D) Immune Globulin is indicated. When antepartum Rh(D) Immune Globulin has been administered, it is possible to detect anti-D in the maternal serum at delivery in as many as 25-30% of women. The infants of these women may occasionally have a positive direct antiglobulin test due to anti-D. The presence of passive anti-D does not preclude administration postpartum. HEELSTICK SPECIMEN: Serological workup on neonates can be done on a heelstick specimen. A neonate is defined as less than four months old. Presence of blood group alloantibodies in neonates is determined on the basis of the antibody screen done on the maternal specimen, within 72 hours of delivery. If the maternal antibody screen was negative, the infant can receive group O, Rh(D) compatible red blood cells without crossmatch or repeated antibody screen for the entire admission. In the event the mother has a significant antibody, a complete major crossmatch is performed utilizing the maternal serum/plasma. If the infant is four months of age or older, the infant must be treated as an adult patient with a new specimen collected and crossmatches done every 3 days. Page 35 The Ohio State University Medical Center Transfusion Service On-Call Manual Blood selection for simple transfusion: 1. Transfuse group O red blood cells, Rh(D) compatible. 2. Red blood cells should be less than 14 days old and must be CMV negative and from a CPDA-1/CPD unit. 3. The red blood cells must be antigen negative for any corresponding maternal antibodies. Mother’s with anti-D due to Rh(D) Immune Globulin- the infant should receive Rh(D) negative blood. A crossmatch is not needed. Blood selection for exchange transfusion: 1. Transfuse group O red blood cells, Rh(D) compatible. 2. Red blood cells should be less than 7 days old, CMV negative, irradiated and from a CPDA-1/CPD unit. 3. Reconstitute with AB fresh frozen plasma to a hematocrit of 50%. To obtain a 50% hematocrit: Weigh the red blood cells and subtract 40 gm for the weight of the collection bag. Multiple by 0.70 to determine the volume of red blood cells per ml. Add 20 ml less of fresh frozen plasma than the volume of red blood cells. 4. Hemoglobin S negative blood will be provided at the physician’s request. Non-red blood cell selection for transfusion: 1. Fresh Frozen Plasma: ABO compatible with the recipient’s red cells. The FFP does not need to be CMV negative and/or irradiated. 2. Platelet: ABO/Rh(D) identical (preferred) or compatible with the recipient’s red cells, as well as, CMV negative and irradiated. BUFFY COAT FOR NEONATES: A buffy coat is a granulocyte concentrate prepared from a single whole blood donation. This component is indicated for neonates with extreme neutropenia plus evidence of sepsis unresponsive to antibiotics. It may be prepared from freshly drawn units of whole blood. The on-call resident pathologist must approve this component. Procurement of the component can be done through the American Red Cross by consultation with the Medical Director (Dr. M. Wissel) or the Chief Executive Officer (Dr. A. Ng). INTRAUTERINE TRANSFUSION: Intrauterine transfusions are undertaken when the fetus is in critical condition due to red blood cell destruction by maternal antibody as shown by a high Delta OD 450 and/or low hemoglobin value seen on cordocentesis. The primary objective is to Page 36 The Ohio State University Medical Center Transfusion Service On-Call Manual correct anemia in the fetus, thus correcting heart failure, hydrops and erythroblastosis. IUT’s are generally repeated on 2-3 week intervals. Donor Red Cell Requirements: 1. Compatible with maternal serum. 2. Group O Rh(D) negative, unless the fetal cells have been typed Rh(D) positive or mother has anti-c or anti-e. 3. Frozen/deglycerolized (especially if mother is a one-time directed donor). 4. CMV negative and irradiated. 5. Hemocrit should be 70% or greater. Determination of IUT volume for transfusion: gestational age – 20 X 10 = volume of donor red cells (ml) KLEIHAUER-BETKE TEST (FETAL HEMOGLOBIN STAIN): This test utilizes the fact that fetal hemoglobin is resistant to acid elution, whereas adult hemoglobin is not. When a thin blood smear is exposed to an citrate phosphate buffer, the adult red blood cells lose their hemoglobin into the buffer so that only the stroma remains. Fetal red blood cells are unaffected and retain their hemoglobin. The percentage of fetal cells in the maternal blood is used to calculate the approximate volume of fetal maternal hemorrhage. This test is performed after the D Rosette Test (Fetal Screen) as a quantitative test for the amount of a fetal-maternal hemorrhage. This value assists in determining the appropriate dose of Rh(D) Immune Globulin to be given to a Rh(D) negative woman. It has also been used in cases of trauma; however, its correlation here is very poor. The Kleihauer-Betke test is performed routinely during dayshift. Between 3:30pm and 8am, the on-call resident pathologist must approve an order for a Kleihauer-Betke. Fetal monitoring is better than Kleihauer-Betke result for trauma (Transfusion 1999, 30:344-357). A Kleihauer-Betke is not performed during the first trimester because the fetal blood volume is <30ml. NOTE: The Fetal Screen test will detect a 5cc red blood cell or 10cc whole blood fetal maternal bleed. NOTE: Results of Kleihauer-Betke are stated as a % of fetal cells seen. Interpretation is based on the following table. Page 37 The Ohio State University Medical Center Transfusion Service On-Call Manual FETAL MATERNAL HEMORRHAGE VOLUME (ML WHOLE BLOOD) % Fetal Cells Range* Average 0.3-0.5 20 <50 0.6-0.8 35 15-80 0.9-1.1 50 22-110 1.2-1.4 65 30-140 1.5-2.0 88 37-200 2.1-2.6 115 52-250 Less than 0.3% fetal cells, give 1 vial of Rh Immune Globulin (IM) Vials Of Rhig (I.M. Preparation) To Inject 2 2 3 3 4 5 *The range provides for the poor accuracy and precision of the Kleihauer-Betke (acid elution) test. These recommendations are based upon 1 vial of Rh Immune Globulin for each 15 ml red blood cells or 30 ml whole blood. Excessive values of fetal hemoglobin are seen in the following: aplastic anemia, sickle cell anemia, erythremic myelosis, Hgb H disease, hereditary spherocytic anemia, hereditary persistence of Hgb F, thalassemia major (40-90% HgbF) and thalassemia minor (5-10% Hgb F). DELTA OD 450 SCAN: The examination of the amniotic fluid for bilirubin-like pigment is a method for evaluating the degree of hemolysis occurring in the infant. The absorbance of the amniotic fluid is measured and plotted on a modified Liley graph as a function of the wavelength. Amniotic fluid from an affected pregnancy will show a characteristic “bulge” in the 450nm area. The Delta OD 450 Scan is run Monday through Friday, 8:00am - 3:30 pm. Interpretation and Recommendations: 1. Zone I: Consistent with an unaffected or mildly affected fetus. 2. Zone IIa: Consistent with a mildly affected fetus. 3. Zone IIb: Consistent with a moderately affected fetus. 4. Zone III: Consistent with a severely affected fetus. Hgb < 8.0 g/dl. Intrauterine transfusion immediately if fetus is at least 20-22 weeks gestation. This test has been almost wholly replaced by the middle cerebral artery peak flow velocity. HLA/PLATELET ANTIBODIES: The chronic platelet user is usually a hematology or oncology patient. When platelet therapy becomes warranted, usually following induction of chemotherapy protocols, the first component used may be a pool of platelet concentrates. Exceptions may include patients with aplastic anemia who, when needed, require platelet apheresis components from the time of diagnosis onward, or other chronically transfused patients. Platelet concentrates are readily available and are the component of choice for many patients. Pre-storage leukocyte-reduction eliminates contaminating white blood cells for Page 38 The Ohio State University Medical Center Transfusion Service On-Call Manual patients who will require components on a chronic, long-term basis. However, leukocytereduction does NOT entirely prevent sensitization. Once a patient is expected to be a chronic platelet user, the patient should be HLA typed. Fifty to seventy percent of these patients will develop HLA/lymphocytotoxic antibodies during their clinical course. HLA antigens are present on platelet surfaces. As such, HLA/lymphocytotoxic antibodies can be the cause of a poor response to platelet transfusion. The HLA typing should be done as early as at the time of the patient’s diagnosis. The patient’s own lymphocytes are used for this test. Again, platelet concentrates can be used first. Apheresis platelets from family members may be preferred by some clinicians. Lack of response to random donor plateletpheresis or platelet concentrates should be documented before crossmatched plateletpheresis is used. HLA-matched plateletpheresis may be effective in the refractory/unresponsive patient. Lack of efficacy is but one part of the indication for crossmatched platelet components. In general, this is manifested by a poor response or lack of response to random platelets at 10 minutes to 1-hour post transfusion on two separate, usually consecutive occasions. This is in the absence of clinical circumstances such as DIC, splenomegaly, sepsis, fever, or hemorrhage. A post transfusion increment, over the patient’s baseline, of less than 7,000 at 10 minutes to 1-hour after transfusion raises the question of refractoriness, in the absence of bleeding or other course for thrombocytopenia. CROSSMATCHED PLATELETPHERESIS: Once it is clear that the patient is refractory to platelet concentrates, a crossmatched plateletpheresis should be considered. Another option is a plateletpheresis drawn from siblings (since there is a 25% chance that a sibling will completely match the recipient). This would purely be a case of transfusing the product and observe the response. HLA-matched products can be considered if crossmatched platelets fail to demonstrate a good response. A list of donors who share similar or cross-reacting HLA antigens is generated on the basis of the patient’s HLA antigen profile determined earlier in the patient’s clinical course. HLA-matched plateletpheresis require the time-consuming process of finding an available, matched donor from the recipient’s HLA list, procuring the component at the American Red Cross, and testing that component for infectious diseases before release for transfusion. Because of this, one needs to anticipate patient needs at least 24 hours in advance when HLA-matched plateletpheresis are being used. Also, there is a need to anticipate patient requirements for the weekend by at least the preceding Thursday in order to easily guarantee a component or in the evaluation of suspected neonatal alloimmune thrombocytopenia (NATP) or post-transfusion purpura (PTP). PLATELET ANTIBODIES: Platelet autoantibodies are associated with idiopathic thrombocytopenia purpura (ITP). The autoantibody test is performed in Flow Cytometry (293-8326) to evaluate the patient’s Page 39 The Ohio State University Medical Center Transfusion Service On-Call Manual own platelets for bound immunoglobulin. This is NOT an essential test in the diagnosis of ITP (Blood 88:3-40, 1996). ITP is a CLINICAL diagnosis with other causes for thrombocytopenia ruled out. Platelet alloantibodies can occur (but rarely do so) after sensitization with antigen (i.e., previous transfusion or pregnancy). This test is performed by the American Red Cross or other lab and is carried out by evaluation of the patient’s serum for platelet-specific antibodies. Heparin-induced thrombocytopenia is a syndrome in which a patient’s platelet count decreases while on heparin. The test for heparin-induced thrombocytopenia is performed in the Coagulation Laboratory (6-6501), Monday through Friday, 7:30am – 2:00pm. Autoantibody tests are indicated for anyone who presents (de novo) with a low platelet count. Heparin thrombocytopenia workup is indicated for anyone who has a low platelet count and who has been treated with heparin. Platelet alloantibody is indicated for the rare patient who does not respond to platelet transfusions despite crossmatched and HLAmatched plateletpheresis or in the evaluation of suspected neonatal immune thrombocytopenia (NATP) or post-transfusion purpura (PTP). AVAILABLE PLATELET COMPONENTS AT THE OSU MEDICAL CENTER Product Procurement HLAmatched Dose Plt. Count Platelet Concentrate From a unit of whole blood No 4 10 x 109 per unit Random plateletpheresis Plateletpheresis from a donor No** 1 bag At least 3 x 1011 per apheresis 200-350 ml per bag Crossmatched plateletpheresis Plateletpheresis from a donor No** 1 bag At least 3 x 1011 per apheresis 200-350 ml per bag Volume 50-60 ml per unit 175-225 ml per dose At least HLA-Matched Plateletpheresis Yes 1 bag 3 x 1011 per apheresis 200-350 per bag plateletpheresis from a donor * All of the above donations are from volunteer donors. Resources are limited; therefore, a protocol for deciding on the proper and indicated platelet components must be followed. **Can be from random donors, family members, or platelet-crossmatch. Page 40 The Ohio State University Medical Center Transfusion Service On-Call Manual APHERESIS ON-CALL PROCEDURE The on-call pathology resident gets first call for therapeutic apheresis requests. The following information is conveyed to the Transfusion Medicine attending on call: 1. Name of patient 2. Medical record number 3. Diagnosis 4. Location 5. Physician contact name and pager The attending may ask the resident to look at peripheral smear. Catheter is available at Transfusion Service front desk. Page 41 The Ohio State University Medical Center Transfusion Service On-Call Manual COAGULATION PARAMETERS PROFILE OF HEMOSTATIC SCREENING TESTSa Hemophilia A, B or Other Intrinsic System Deficiency or Inhibitor Von Severe Willebrand Heparin Acute DIC Disease Warfarin Final Common Factor VII Pathway Deficiency Deficiency or Inhibitor or Inhibitor Hypofibrinogenemia or Chronic DysfibroLiver genemia Disease Plt Count Normal Normal Low Normal Normal Normal Normal Normal Normal Bleeding time Normal Prolonged Prolonged Normal Normal Normal Normal Normal Normal Fibrinogen (Kinetic Method) Normal Normal Low Normal Normal Normal Low or normal Low or normal Thrombin time Normal Normal Prolonged Prolonged Normal Normal Normal Prolonged Normal or prolonged Repilase time Normal Normal Prolonged Normal Normal Normal Normal Prolonged Normal or prolonged PT Normal Normal Prolonged Normal or Prolonged Prolonged Prolonged Prolonged Prolonged Prolonged or normal PTT Prolonged Prolonged Prolonged Prolonged Prolonged Prolonged Normal Prolonged Prolonged Normal Adapted from Rossi EC, et al. Principles of Transfusion Medicine. Williams and Wilkens: Baltimore, MD: 2nd Edition, 1996. LABORATORY TESTS AND HEMOSTATIC DISORDERS Bleeding Time PT Platelet Count Possible Defects aPTT Fibrinogen VIII, IX, XI, heparin II, V, X, dysfibrinogenemia, heparin, malnutrition, warfarin Abnormal Abnormal Normal Abnormal Normal Normal Normal Normal Normal Normal von Willebrand’s disease Afibrinogenemia, hyperfibrinolysis Thrombocytopenia Qualitative platelet disorder (aspirin, other drugs, thrombopathy Normal Abnormal Normal Normal Abnormal Normal Normal Normal Normal Abnormal Abnormal Abnormal Normal Normal Abnormal Normal Normal Low Normal Normal Factor XIII DIC, severe liver disease HMW Kininogen, prekalikrein, factor XII, lupus anticoagulanta Normal Abnomral Abnormal Normal Abnormal Normal Normal Abnormal Normal Normal Abnormal Normal Normal Abnormal Normal aThe appropriate sequence of screening tests differs for critically ill patients with acquired bleeding defects and for patients who are seen for evaluation of possible mild congenital disorders. Late-stage defects with hypofibrinogenemia and delayed fibrin polymerization from degradation components are common in critically ill patients, while intrinsic system defects and platelet function abnormalities are common congenital disorders. Patients with absence of a 2plasma inhibitor or factor XIII will have excess bleeding despite normal screening tests. Clot solubility in 5 M urea is abnormal in factor XIII deficiency, and patients with a2-plasmin inhibitor deficiency will have a short clot lysis time. Adapted from Rossi EC, et al. Principles of Transfusion Medicine. Williams and Wilkens: Baltimore, MD: 2nd Edition, 1996. Page 42 The Ohio State University Medical Center Transfusion Service On-Call Manual TYPICAL LABORATORY ABNORMALITIES IN THE BLEEDING SURGICAL PATIENTa Basic Laboratory Screen Normal Ranges Massive transfusion DIC Drugs Aspirin Vitamin K deficiency Heparin Uremia Heparin failure CPB Factor deficiencies Platelet Count 250,000400,000/l Bleeding Time <8-10 min PT aPTT 11-13 sec 25-35 sec nl nl nl Mild nl Mild nl nl nl nl/ nl/ nl/ Mild Mild >75,000 nl Ancillary Laboratory Tests Thrombin Fibrinogen FDP Time <12 25010 sec g/ml 400 mg/dl nl/ nl/ Repilase Time nl/ >40g/nl nl/ nl nl nl/ nl/ nl/ nl nl/ nl/ nl nl nl nl nl Abbreviations and symbols: nl - normal; , significantly prolonged, CPB - cardiopulmonary bypass; - significantly decreased. Adapted from Rossi EC, et al. Principles of Transfusion Medicine. Williams and Wilkens: Baltimore, MD,: 2nd Edition, 1996. HEMOSTATIC DEFECTS IN PATIENTS WITH LIVER DISEASE Screening Test Defect aEtOH, Potential Mechanism(s) Platelet Count Folate deficiency Toxic effects of EtOHa Splenic pooling Viral suppression of production DIC (concomitant) Bleeding time (mild) Fribrinogen) degradation components ? Bilirubin EtOH PT and PTT Vitamin K-dependent carboxylation Protein (factor) synthesis Fibrinogen DIC (concomitant) Production (fulminant disease) Thrombin time Dysfibrinogen ( sialic acid) Fibrin degradation components Alcohol Adapted from Rossi EC, Et al. Principles of Transfusion Medicine. Williams and Wilkens: Baltimore, MD; 2nd Edition, 1996. Page 43 The Ohio State University Medical Center Transfusion Service On-Call Manual COAGULATION FACTOR REPLACEMENT Deficient factor t1/2 in vivo I 3-4 d Factor level adequate for hemostasis 100 mg/dL 1, 1, p p 1033 Product name or abbreviationa Loading dose Maintenance dose Cryoprecipitate 1 bag/7 kg 4, p 1 bag/15 kg qd 8, 756; k p 1108; k FFP, cryopoor plasma 3, p 24 10-20 mL/kg 4, 3 mL/kg q 24 h 1, p 756; 1, p 841 p 841 Factor IX Complex (Profilnine SD [Alpha Therapeutic Corp.]) FFP, cryopoor plasma 3, p 24 20 IU/kg 6, m 5 IU/kg q 24 h 6, m 20 mL/kg 1, p 3-6 mL/kg q 12 h 843 1, p 843 FFP, cryopoor plasma 3, p 24 10-20 mL/kg 4, 20 mL/kg q 4 h or 40 mL/kg q 8 h 14, 1033 II 2-5 d 20-40% 1, p 1, p 1033 1033 V 1536 h 25-30% 1, p 843 1, pp 843, 1033 VII 4-7 h 10-20% 1; pp 1, p 845, 1033 p 756; j 1033 VIII 9-18 h 1; pp b 100%d 819, 1033 Factor VIIa (Recombinant) (NovoSeven [Novo Nordisk]) Antihemophilic Factor (Human) (MONARC-M [American Red Cross]), Antihemophilic Factor (Recombinant) (Helixate FS [Aventis Behring], Recombinate [Baxter]) Cryoprecipitate 15-30 mcg/kg 16 50 IU/kgc 50 IU/kg q 12 h, or 50 IU/kg/12 h by continuous infusionc 0.5 bag/kg 3, pp 0.5 bag/kg q 12 he 50 IU FVIII:C/kg q 12 h, or 50 IU FVIII:C/kg/12 h by continuous infusionc 26-27 IX 2024 h 1; pp 819, 1033 100%d Antihemophilic Factor/von Willebrand Factor Complex (Human) (Humate-P [Aventis Behring GmbH]) Factor IX Complex, Factor IX (Recombinant) (BeneFIX [Wyeth]), Factor IX (Human) (AlphaNine SD [Alpha Therapeutic Corp.]) Page 44 15-30 µg/kg q 46 h 16 50 IU FVIII:C/kgc 100 IU/kgc 50 IU/kg q 12 hc The Ohio State University Medical Center Transfusion Service On-Call Manual Deficient factor t1/2 in vivo X 2042 h Factor level adequate for hemostasis 10-40% 1, p Product name or abbreviationa Loading dose Maintenance dose Factor IX Complex (Profilnine SD [Alpha Therapeutic Corp.]) FFP, cryopoor plasma 3, p 24 FFP, cryopoor plasma 3, p 24 10-20 IU/kg 4, 8-10 IU/kg q 24 h p 756; n 10, n 10-20 mL/kg 8, 3-6 mL/kg q 12 h p 212 8, p 212 Not applicable Not applicable Not applicable Not applicable 1-5%g Cryoprecipitate 1, p 1 unit/10-20 kgf 1 unit/10-20 kg q 3-4 wk 12 FFP 500 mL 8, p 1107 Antihemophilic Factor/von Willebrand Factor Complex (Human) (Humate-P [Aventis Behring GmbH]) Antihemophilic Factor (Human) (Alphanate [Alpha Therapeutic]) Cryoprecipitate 60-80 IU VWF:RCo/kg 2-3 mL/kg q 4-6 wk 12, h 40-60 IU VWF:RCo/kg q 8-12 h 1, p 833 848 1, p 848 XI 4080 h 15-25% 1, p 1033 10-20 mL/kg 4, p 756 5 mL/kg q 12-24 h 12 1, p 1033 XII 4852 h 1, p 1033 XIII 12 d 1, p 238 1033 Von Willebrand Factor (VWF) 3-5 h 25-50% 1, p 5, p 27 1033 1, p 833 60-70 IU Factor VIII:C/kgi 40-50 IU Factor VIII:C/kg q 12 h 1, 1-2 bags/10 kg 1, p 832 0.7-1.4 bags/10 kg q 8-12 hi p 832 Notes a Product names are from references 17 and 18. Abbreviation Cryopoor plasma Cryoprecipitate FFP Proper name 17, 18 Plasma, Cryoprecipitate Reduced Cryoprecipitated AHF Fresh Frozen Plasma Pooled Plasma, Solvent/Detergent Treated, may be substituted for Fresh Frozen Plasma (FFP). b Other recommendations: 5 mL/kg q 6-24 h 12, 5 mL/kg q 6 h 15, 10-15 mL/kg q 3-4 d 11. c For the most serious bleeding. See reference 1, p 819, for details. d 100% for CNS, traumatic, surgical, or retroperitoneal bleeding; less for less severe bleeding. See reference 1, p 819, for details. e The maintenance dose is the same as the loading dose 1, p 819. Page 45 The Ohio State University Medical Center Transfusion Service On-Call Manual f The loading dose of Factor XIII concentrate recommended by Giangrande was about the same as the maintenance dose he recommended. Thus, the loading dose of Cryoprecipitated AHF has been set to be the same as the maintenance dose. g 1-2% 1, p 238, 3-5% 12. But reference 12 recommends 25-50% following major trauma. h An alternate recommendation of 500 mL q 2 d 8, p 1107 appears to be inconsistent with the half-life of Factor XIII as well as with the recommendations of references 12 and 20. i Based on the ratio of maintenance to loading doses for VWF:RCo 1, p 833. j Assuming that the product contains 1 IU/mL 3, p 22. k Each bag has about 250 mg of fibrinogen on average. 4, p 467 m Calculate volume of Profilnine SD using the estimate of 1.2 IU of factor II per IU of factor IX. 19 n Calculate volume of Profilnine SD using the estimate of 0.6 IU of factor X per IU of factor IX. 19 References 1. Colman RW et al, eds. Hemostasis and thrombosis: basic principles and clinical practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2001. 1578 pp. 2. Giangrande PLF. Factor XIII deficiency. Oxford Haemophilia Centre. http://www.medicine.ox.ac.uk/ohc/xiii.htm, as seen on 12/21/01. 3. American Association of Blood Banks, America's Blood Centers, American Red Cross. Circular of information for the use of human blood and blood components. http://www.aabb.org/All_About_Blood/COI/coiv2.pdf. 2000. 4. Technical manual. 13th ed. Bethesda: American Association of Blood Banks, 1999. 798 pp. 5. Blood transfusion therapy: a physician's handbook. 6th ed. Bethesda: American Association of Blood Banks, 1999. 150 pp. 6. Lechler E. Use of prothrombin complex concentrates for prophylaxis and treatment of bleeding episodes in patients with hereditary deficiency of prothrombin, factor VII, factor X, protein C, protein S, or protein Z. Thromb Res 1999;95:S39-S50. 7. Practice guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732-747. 8. Colman RW et al, eds. Hemostasis and thrombosis: basic principles and clinical practice. 3rd ed. Philadelphia: JB Lippincott Co, 1994. 1713 pp. 9. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. JAMA 1994;271:777-81. 10. Larrain AC. Deficiencia congénita en factor X de la coagulación sanguínea. Resultado exitoso del empleo de concentrados de complejo protrombinico en el control de la tendencia hemorrágica en operación cesárea en 2 embarazos [Congenital blood coagulation factor X deficiency. Successful result of the use of prothrombin concentrated complex in the control of cesarean section hemorrhage in 2 pregnancies]. Rev Med Chil 1994;122:1178–83. 11. Marder VJ, Shulman NR. Clinical aspects of congenital factor VII deficiency. Am J Med 1964;37:182-94 Page 46 The Ohio State University Medical Center Transfusion Service On-Call Manual 12. 13. 14. 15. 16. 17. 18. 19. 20. Rodgers GM, Greenberg CS. Inherited coagulation disorders. In: Lee GR et al, eds. Wintrobe's clinical hematology. 10th ed. Baltimore: Williams & Wilkins, 1999:1682-1732. Horowitz MS, Pehta JC. SD Plasma in TTP and coagulation factor deficiencies for which no concentrates are available. Vox Sang 1998;74 Suppl 1:231-5 Saint-Raymond S, Greffe B, Carré J, et al. Attitude pratique en cas d'intervention chirurgicale chez un sujet atteint d'un déficit congénital en facteur VII [Practical approaches for surgical procedures in congenital factor VII deficiency]. Ann Fr Anesth Réanim 1989;8:518-521 Caldwell DC et al. Clinics in Obstetrics and Gynecology 1985;28(1):53-72. Cited in: Fadel HE, Krauss JS. Factor VII deficiency and pregnancy. Obstet Gynecol 1989;73(3 Pt 2):453-454 Scharrer I. Recombinant factor VIIa for patients with inhibitors to factor VIII or IX or factor VII deficiency. Haemophilia 1999; 5:253-259 USP DI. Drug information for the health care provider. Rockville, MD: United States Pharmacopeial Convention, 2002. Code of Federal Regulations, Title 21--Food and Drugs, Part 640--Additional standards for human blood and blood products. Gross, John, RN, MS; Director, Professional Services; Alpha Therapeutic Corp. Email to Barry Siegfried, MD; 5/10/02. Board PG et al. Factor XIII: inherited and acquired deficiency. Blood Rev 1993;7:229-242. Page 47 The Ohio State University Medical Center Transfusion Service On-Call Manual BLOOD COMPONENTS AND PLASMA DERIVATIVES Component/Product Whole Blood Composition RBCs (approx. Hct 40%); plasma WBCs; PLTS Volume 500 mL Indications Increase both red cell mass and plasma volume (WBCs and PLTS not functional; plasma deficient in labile clotting Factors V and VIII) Red Blood Cells RBC (approx. Hct 75%); Reduced plasma, WBCs, and PLTS 250 mL Increase red cell mass in symptomatic anemia (WBCs and PLTS not functional) Red Blood Cells, Adenine-Saline Added RBC (approx. Hct 60%); Reduced plasma, WBCs and PLTS; 100 mL of additive solution >85% original volume of RBC; <5 x 106 WBCs; few PLTS; minimal plasma 330 mL Increase red cell mass in symptomatic anemia (WBCs and PLTS not functional) 225 mL Increase red cell mass; <5 x 106 WBCs to decrease the likelihood of febrile reactions, immunization to leukocytes (HLA antigens) or CMV transmission RBCs Washed RBCs (approx. Hct 75%); <5 x 108 WBCs; no plasma 180 mL Increase red cell mass; reduce risk of allergic reactions to plasma proteins RBCs Frozen; RBCs Deglycerolized RBC (approx. Hct 75%); <5 x 108 WBCs; no PLTS; no plasma 180 mL Increase red cell mass; minimize febrile or allergic transfusion reactions; use for prolonged RBC blood storage Granulocytes Pheresis Granulocytes (>1.0 x 1010 PMN/unit; lymphocytes; PLTS (>2.0 x 1011/unit); some RBCs PLTS (>5.5 x 1010/unit); RBC; WBCs; plasma 220 mL Provide granulocytes for selected patients with sepsis and severe neutropenia (<500 PMN/L) 50 mL Bleeding due to thrombocytopenia or thrombocytopathy PLTS (>3 x 1011/unit; RBCs; WBCs; plasma PLTS (as above); <5 x 106 WBCs per final dose of pooled PLTS 300 mL Plasma; all coagulation factors; complement (no PLTS) Fibrinogen; Factors VIII and XIII; von Willebrand factor 220 mL Same as PLTS; sometimes HLA matched or platelet crossmatched Same as PLTS; <5 x 106 WBCs to decrease the likelihood of febrile reactions, allo-immunization to leukocytes (HLA antigens) or CMV transmission Treatment of some coagulation disorders Factor VIII (concentrates, recombinant human Factor VIII) Factor IX (concentrates, recombinant human Factor IX) Albumin/PPF Factor VIII; trace amount of other plasma proteins (products vary in purity) 25mL Factor IX; trace amount of other plasma proteins (products vary in purity) 25 mL Hemophilia B (Factor IX deficiency) Albumin, some -, globulins Volume expansion Immune Globulin IgG antibodies; preparations for IV and/or IM use (5%); (25%) varies Rh Immune Globulin IgG anti-D; preparations for IV and/or IM use RBCs Leukocytes Reduced (prepared by filtration) Platelet Platelets Pheresis Platelets Leukocytes Reduced FFP; DR-P; SD-P Cryoprecipitated AHF 300 mL 15 mL varies Deficiency of fibrinogen; Factor XIII; second choice in treatment of Hemophilia A, von Willebrand disease Hemophilia A (Factor VIII deficiency); von Willebrand’s disease (off-label use for selected products only) Treatment of hypo- or agammaglobulenemia; disease prophylaxis; autoimmune thrombocytopenia (IV only) Prevention of hemolytic disease of the newborn due to D antigen; treatment of autoimmune thrombocytopenia; Transfusion Accidents RBCs = red blood cells; Hct = hematocrit; WBCs = white blood cells; PLTS = platelets; CMV = cytomegalovirus; PMN = polymorphonuclear cells; FFP = fresh frozen plasma; PPF = plasma protein fraction; IV = intravenous; IM = Intramuscular; DR-P = donor rested plasma; SD-P = solvent/detergent-treated plasma Page 48 The Ohio State University Medical Center Transfusion Service On-Call Manual BLOOD TRANSFUSION THERAPY DATA-CARD® ADMINISTRATION OF BLOOD COMPONENTS A. Positive identification of the recipient to be transfused and the designated blood unit to be used is essential. B. Compatibility of red blood cells with IV solutions: 1. Use 0.9% Sodium Chloride for injection, USP. 2. DO NOT use 5% Dextrose solutions (may induce hemolysis). 3. DO NOT use Lactated Ringer’s (contains Ca++ which may induce clot formation in the blood bag and/or administration set). 4. Add NO medications to blood. 5. Plasma (type compatible) or Albumin (5%) is acceptable in special circumstances. C. Blood Warming: Use only a temperature-monitored blood warmer to avoid hemolysis. Indications for blood warming: 1. Adults receiving blood over 50ml/kg/hour. 2. Children receiving blood over 15ml/kg/hour. 3. Patients with clinically active cold agglutinins. 4. Rapid infusion of blood through central lines (cold blood may induce arrhythmias). D. Blood Filters. ALL BLOOD COMPONENTS MUST BE INFUSED THROUGH A FILTER. 1. Use a standard blood filter (170-260 microns screen) for all blood components. 2. Leukocyte-reduction filters are used to: a. Decrease febrile transfusion reactions. b. Decrease risk of alloimmunization to leukocyte or HLA antigens. c. Reduce CMV transmission (proper technique is critical). E. Time Limit for infusion. Components should be infused within 4 hours. Note: the blood bank can divide components into aliquots as needed. F. Irradiation of Blood and Cellular Components. A minimum of 25 Gy should be used to reduce the risk of TA-GVHD in susceptible patients such as: selected immunoincompetent or immuno-compromised recipients (i.e., hematopoietic progenitor transplant, congenital immune deficiency, etc.), a fetus receiving intrauterine transfusions, recipients of donor units from blood relatives, recipients of HLA-matched platelets. Darrell J. Triulzi, MD(ed) Copyright © 1981, 1993, 1996, 1999 American Assocation of Blood Banks, 8101 Glenbrook Road, Bethesda, MD 20814 Page 49 The Ohio State University Medical Center Transfusion Service On-Call Manual RED BLOOD CELLS (RBC) Red blood cells remaining after separating plasma from human blood by centrifugal or gravitational force. Volume: CPD: CPDA-1: Volume Hct 250-300 ml 80% 250-300 ml 80% AS-1: 350-400 ml 55-65% Action: 1. Increases oxygen-carrying capacity. 2. Increases RBC/mass for volume replacement. Indications: 1. Improve Hgb/Hct to increase oxygen-carrying capacity. 2. Replacement of blood loss (i.e., trauma, surgical procedures, hemorrhage). 3. Clinical use: anemia, cardiac patients, liver and kidney disease. Contraindications: 1. Do not use when anemia can be corrected with specific medications. 2. Do not use for correcting coagulation deficiencies. Side Effects & Hazards: 1. Hemolytic transfusion reaction 2. Infectious disease transmission 3. Graft versus Host response 4. Alloimmunization 5. Febrile reactions 6. Circulatory overload 7. Iron overload 8. Allergic reactions 9. Bacterial contamination 10. Microaggregates 11. Air embolism 12. Metabolic complications 13. Coagulation proteins and platelet depletion (if a massive transfusion) Dosage: Depends on clinical condition. Should raise patient’s hematocrit 3% or 1 gm/dl/unit. Page 50 The Ohio State University Medical Center Transfusion Service On-Call Manual Rate of Infusion: Depends on clinical condition. No slower than four hours per unit. Storage: 1-6C Shelf life: CPD: 21 days CPDA-1: 35 days AS-1: 42 days Administration: 1. Must be ABO and Rh(D) compatible (crossmatch required). 2. Y-type tubing with blood filters; 0.9 NaCl prn. (50-100 ml). 3. If clinically indicated, blood may be warmed (not to exceed 42C). For additional information refer to Circular of Information for the Use of Human Blood and Blood Components. CPD QUADS AND BABY SPECIALS (FILTERED) Baby specials and quads are CMV negative O positive or O negative red blood cells intended for neonatal transfusions. The baby special is a CPD packed cell that has 4 satellite bags attached. Volume: CPD packed cell: Hct: Action: 250-300 ml 60-75% Same as for Red Blood Cells Indications: 1. Improve Hgb/Hct to increase oxygen-carrying capacity. 2. Exchange transfusion. Side Effects and Hazards: 1. Similar to those indicated for Red Blood Cells. Dosage: 2. Depends upon clinical conditions. 5-10 ml/kg or 1 unit/35 kg. Rate of Infusion: 1. No slower than 4 hours per aliquot. Storage: 1-6C Shelf life: 1. CPD: 21 days. These red blood cells will be <14 days old. Page 51 The Ohio State University Medical Center Transfusion Service On-Call Manual Administration: 1. Must be ABO and Rh(D) compatible. 2. Y-type tubing with blood filters; 0.9 NaCl prn. 3. If clinically indicated, blood may be warmed (not to exceed 42C). RED BLOOD CELLS, FILTERED The high efficiency leukocyte removal (3rd generation leukocyte-depletion filter) traps a large number of leukocytes and allows for excellent red cell recovery. Volume: <4.0 x 105 WBC remaining (ARC) 93% RBC recovery (ARC) 55% Hct (plasma similar to PRC) Action: Same as for Red Blood Cells. Indications: 1. Febrile transfusion reactions 2. Delay of leukocyte alloimmunization 3. Chronic dialysis patients who are transplant candidates 4. Aplastic anemia, patients anticipating bone marrow transplantation 5. Patients with PNH (actually preferred over deglyc’ed/washed RBC’s). 6. To prevent transfusion-induced immunosuppression and potentially prevent transfusion-induced CMV infection 7. All patients? Contraindications: Same as for Red Blood Cells. Side Effects and Hazards: Similar to those indicated for Red Blood Cells. Dosage: Depends on clinical condition. Rate of Infusion: Storage: No slower than four hours per unit. 1-6C Shelf life: Original outdate if filtered in-line prestorage. Administration: 1. Must be ABO and Rh(D) compatible. 2. Y-type blood recipient set; 0.9 NaCl prn. Page 52 The Ohio State University Medical Center Transfusion Service On-Call Manual 3. If clinically indicated, blood may be warmed (not to exceed 42C). For additional information, refer to Circular of Information for the Use of Human Blood and Blood Components. RED BLOOD CELLS DEGLYCEROLIZED These are red cells primarily frozen with glycerol (storage over 10 years). They are usually of rare antigen phenotype. Volume: 80% RBC recovery. Action: Same as for Red Blood Cells. Indications: 1. Patients with high frequency antibodies requiring rare blood. 2. Patients with multiple antibodies requiring rare blood. 3. Patient with severe febrile reactions unresponsive to filtered/leuko-depleted blood. 4. Patients exhibiting excessive reactions to plasma (PNH, IgA sensitization and IgA deficiency [although washed RBC’s are less expensive and have a faster turnaround time]). 5. Autologous predeposit of red cells, rarely. 6. Intrauterine transfusion. Contraindication: Same as for Red Blood Cells. Side Effects and Hazards: Similar to those indicated for Red Blood Cells. Dosage: Depends on clinical condition. Rate of Infusion: No slower than four hours per unit. Storage: 1-6C Shelf life: 24 hours after thawing. Administration: 1. Must be ABO and Rh(D) compatible. 2. Y-type blood recipient set; 0.9 NaCl prn. a. Two units of deglycerolized cells, requiring no special antigen typing, can be completed and available from the American Red Cross in approximately 1½ hours. This is an approximate time, which is dependent on the American Red Cross workload. Page 53 The Ohio State University Medical Center Transfusion Service On-Call Manual b. Two units of deglycerolized cells, requiring special antigen typing, can be completed and available from American Red Cross in approximately 4 hours. This does not include Reference Laboratory consultation, if needed. This time is an approximate time dependent on the American Red Cross workload. For additional information, refer to Circulation of Information for the Use of Human Blood and Blood Components. PLATELETS Platelets collected from one unit whole blood donation and suspended in a specific volume of original plasma. Volume: 45-65 ml plasma containing a minimum 5.5 x 10 10 platelets (AABB Standards). The Central Ohio American Red Cross 1996, average 9-10 x 1010. Action: Corrects hemostatic deficit. Used most to treat hemorrhage, but also (in practice) to elevate the platelet count prophylactically: 1. Patients with thrombocytopenia or an abnormality of platelet function or both who have significant bleeding should receive platelets if the platelet disorder is likely to be causing or contributing to the bleeding. 2. Prophylaxis for patient with severe thrombocytopenia (<10 to 20,000/l). 3. Patients given massive transfusions in which dilutional thrombocytopenia results. 4. Uremia with bleeding or invasive procedure (after DDAVP or dialysis or cryoprecipitate), cardiopulmonary bypass. 5. Medications decreasing platelet function. 6. Prior to an invasive procedure or surgery when platelet count is <50,000. Contraindications: 1. Do not use if bleeding is unrelated to thrombocytopenia or abnormality functioning platelets. 2. Rapid platelet destruction: ITP (except in acute hemorrhage). 3. Contraindicated in TTP, except in acute hemorrhage or surgery. Side Effects and Hazards: 1. Infectious disease transmission 2. Bacterial contamination 3. Allergic reactions 4. Febrile reactions 5. Circulatory overload 6. Air embolism Page 54 The Ohio State University Medical Center Transfusion Service On-Call Manual 7. Graft versus Host response 8. Alloimmunization 9. Hypotension reactions, cytokine associated Dosage: 1. Depends on clinical situations. 2. One unit of platelet concentrate usually increases the platelet count by 10,00015,000/l in a 70-kg adult and 20,000 or more in an 18-kg child. 3. The usual dose in a patient with bleeding symptoms of a platelet count below 20,000 l is 4 units. 4. Pooled platelet concentrates, 4 platelets concentrates (200 ml). 5. Pediatrics: 5-10cc/kg or dose of 1 x 1011/kg. Rate of Infusion: approximately 60 minutes per pool. Storage: 20-24C with continuous gentle agitation. Shelf life: 5 days Administration: 1. Platelet ABO incompatible with the recipient’s plasma may be used if not grossly contaminated with red blood cells (crossmatching not required if less than 2ml of RBC), although ABO identical strongly encouraged. 2. Blood component infusion set. 3. 0.9 NaCl prn. NOTE: The container and filter may be flushed with normal saline to maximize the number of platelets administered. NOTE: Less than 2,500 platelet count increase per unit transfused equates to a refractory state. Alloimmunization should be considered with a corrected count increment (CCI) less than 7,500, drawn 10 minutes to 1 hour post-transfusion. CCI = platelet increment X body surface area divided by the number of platelets (X 1011) One platelet concentrate is equal to at least 0.55 X 10 11 platelets. (Central Ohio American Red Cross average .9-1 x 1011) For additional information, refer to Circular of Information for the Use of Human Blood and Blood components. Page 55 The Ohio State University Medical Center Transfusion Service On-Call Manual PLATELETPHERESIS The separation and retention of platelets Plateletpheresis Guidelines 1. CMV-negative irradiated single-donor platelet: (with or without HLA-matching or platelet crossmatching) For bone marrow transplantation program patients (excluding CMV positive autologous BMT) at OSU as decided by the BMT team. 2. Crossmatched or HLA-matched single donor platelet: Reserved for thrombocytopenic patients who have demonstrated evidence of alloimmunization to platelets and have become refractory to random-donor platelet concentrates. The following sequential steps should be followed by the physician attempting to optimize platelet support for needy patients. a. Thrombocytopenia and prophylactic platelet transfusion: i. Platelet count <10,000 in stable patients. ii. Platelet count <20,000 in high risk patients iii. Platelet count <50,000 in patients with invasive procedure or clinical bleeding. b. Alloimmunization: i. Evidence is established when a 10 minute to 1 hour corrected count increment is less than 7,500. ii. HLA-antibody or platelet antibody screening is positive and may indicate alloimmunization (see iv). iii. Without other clinical factors of platelet destruction, namely, DIC, sepsis, fever, splenomegaly, brisk hemorrhage, or drug-induced antibody; then platelet-crossmatched or HLA-matched products can be tried when (i) and (ii) are satisfied. iv. If there are clinical factors of platelet destruction, then a 10-minute posttransfusion C.C.I. should be done subsequent to the next random-donor platelet transfusion. If this is <7.500, then platelet-crossmatched or HLAmatched platelet support is a reasonable and productive means of managing the patient. v. A 1-hour post-transfusion C.C.I.* is an essential means of monitoring the outcome of the transfusion and will help in the selection of best donors. c. Availability of Components: i. Due to the donor’s need for advance notice, we strongly recommend all orders be written 24 hours in advance. Every attempt will be made to fill orders. ii. Emergencies will be expedited as much as possible. *C.C.I. = Platelet Increment x Body Surface Area (sq.m.) number of platelets transfused (1011) Page 56 The Ohio State University Medical Center Transfusion Service On-Call Manual Platelet Increment is Pre-transfusion Platelet Count – Post-Transfusion Platelet Count The post-transfusion count should be done at 10 minutes to 1 hour and 24 hours* Number of platelets transfused (1011) = 0.9 x number of platelet concentrate (assuming 1 platelet concentrate = 0.9 x 1011 platelets); 3.0 for apheresis platelet Volume: 200-300 ml 3-4 x 1011 platelets (1996, ARC) Storage: Store at room temperature (22C) using gentle agitation. Shelf life: 5-day storage. Administration: 1. Usually will be ABO and Rh(D) compatible. 2. Y-type blood component recipient set and 0.9 NaCl prn. 3. Infuse as quickly as possible. Plateletpheresis – Single Donor – 5-day Expiration: 1. For split platelet compoents, contents from the two bags should be pooled into the labeled bag before transfusion and must be transfused within 24 hours after pooling (or sooner if the 5-day period elapses before then). 2. Store at 20C-24C with agitation. Preferably use a rotator, which operates at 70 strokes per minute with the length of the stroke being 1½ inches. 3. Compatibility testing is not required for administration of product, unless pheresis contains >2 ml red blood cells. 4. Administer over 60 minutes through component recipient set. For additional information, refer to Circular of Information For the Use of Human Blood and Blood Components. FRESH FROZEN PLASMA (FFP) Single donor plasma frozen within 8 hours of collection and stored at –18C or colder. Volume: greater than 150 ml (ARC) 200 units Factor VIII/unit 400 mg Fibrinogen/unit ~200 units of all coagulation factors Action: 1. Provides coagulation factors, anti-thrombotic factors, other plasma proteins and enzymes (i.e., C1-esterase inhibitor). Page 57 The Ohio State University Medical Center Transfusion Service On-Call Manual Indications: 1. Patients with coagulation protein deficiency for which specific factor concentrates are unavailable or undesirable (II, V, VII, X and XI). 2. Dcumented coagulation defects during or after massive transfusion. 3. Reversal of warfarin effect in patients who are bleeding or who will be undergoing an invasive procedure (holding dose on vitamin K preferred). 4. Multiple coagulation defects as in liver disease. 5. Therapeutic plasma exchange for thrombotic thrombocytopenic purpura (second line). Contraindication: 1. Do not use when coagulopathy can be corrected with specific therapy (i.e., Vitamin K and waiting 24 hours for an effect). 2. FFP should not be used as a volume expander nor as a nutritional source since safer and more appropriate therapies exist. Hazards: 1. Allergic reactions + anaphylaxis) 2. Febrile reactions/TRALI 3. Circulatory overload 4. Citrate toxicity, hypothermia and other metabolic problems with large volume. 5. Infectious disease transmission Dosage: 1. Depends on the clinical situation and may be determined by serial laboratory assays of coagulation function (PT, PTT). 1 FFP may increase all coagulation factors 6-8%. 2. Bleeding: 13-15 ml/kg (i.e., 2-4 FFP in one dose) Neonatal children: 13-15 ml/kg. 3. 15-20mg/dl increase in fibrinogen per unit. Rate of Infusion: Approximately 5-10ml per minute. Storage: 1. –18C or colder (1 year); STORE FLAT. 2. 1-6C AFTER THAWING. Shelf Life: 1. 1 year in frozen state. Page 58 The Ohio State University Medical Center Transfusion Service On-Call Manual 2. 24 hours after thawing. 3. 5 days as thawed plasma. Administration: Patient type Can Receive FFP of Type O A, B, AB, O A A, AB B B, AB AB AB 1. Must be ABO compatible, Rh(D) type need not be a consideration. 2. Thaw product between 30-37C with gentle agitation. 3. Use Y-type or straight line set with component filter. For additional information, refer to Circular of Information for the Use of Human Blood and Blood Components. Emergency Replacement Therapy with Plasma for Miscellaneous Specific Coagulation Deficiencies Disease Hemophilia A (factor VIII deficiency) Loading Dose Not required Maintenance Dose 15 ml/kg every 8 hours for 12 days and every 12 hours thereafter Hemophilia Ba (factor IX deficiency) 60 ml/kg 7 ml/kg every 12 hours von Willebrand’s diseasea Not required 10 ml/kg daily Prothrombin deficiency 15 ml/kg 5-10 ml/kg daily Factor V deficiency 20 ml/kg 10 ml/kg every 12 hours Factor VII deficiency 10 ml/kg 5 ml/kg every 6-24 hours Factor X deficiency 15 ml/kg 10 ml/kg daily Factor XI deficiency 10 ml/kg 5 ml/kg daily Factor XIII deficiency 5 ml/kg every 1-2 weeks Not Required aSpecific factor concentrates are available and more effective and convenient than plasma in most situations. (From Wintrobe MM. Therapy of The Hereditary Coagulation Disorders. In: Clinical Hematology. Philadelphia: Lea & Febiger, 1979:1190). Page 59 The Ohio State University Medical Center Transfusion Service On-Call Manual CRYO POOR PLASMA/CRYO SUPERNATANT LIQUID PLASMA Fresh frozen plasma is thawed at 4C and the cryoprecipitate forms. The liquid supernatant plasma is separated from the cryoprecipitate and then refrozen. Volume: 200-250 ml Indications: 1. Infusion of cryosupernatant is used for the treatment of patients with thrombotic thrombocytopenia purpura. This disease is characterized by the development of fever, thrombocytopenia, hemolytic anemia, renal failure and CNS disturbance. Many patients with TTP respond satisfactorily to fresh frozen plasma given as an infusion or as plasma replacement with plasmapheresis. However, patients with TTP have been reported to respond to treatment of cryosupernatant. The pathophysiology of TTP is a matter of ongoing research. However, evidence indicates that platelets and large multimeric forms of VWF are consumed in association with intravascular platelet aggregation. Therefore, it is postulated that plasma depleted of large multimers of VWF would be preferable in the treatment of TTP. Cryopoor plasma can be used either as first-line therapy or after failure of FFP for TTP. Precautions: 1. Cryosupernatant will have reduced amounts of FVIII:C, FVIII:VWF, fibrinogen, FXIII and fibronectin and should not be used for patient who require therapeutic doses of these factors. Dose: 1. Dependent on the clinical response of the patient. Storage: 1. –18C (1 year); store flat 2. 1-6C after thawing (24 hours) Side Effects and Hazards: 1. Similar to those indicated for fresh frozen plasma. Administration: 1. Must be ABO compatible, Rh(D) type need not be a consideration. 2. Thaw product between 30-37C with gentle agitation. 3. Use Y-type tubing with component filter or straight line set with component filter. For additional information, refer to Circular of Information for the Use of Human Blood and Blood Components. Page 60 The Ohio State University Medical Center Transfusion Service On-Call Manual CRYOPRECIPITATE AHF A concentrated source of fibrinogen, FVIII:C, VWF and FXIII obtained from a single unit of fresh frozen plasma. Volume: 10-15 ml/unit (ARC) Minimum Factor VIII Activity: 80 units/bag. ARC Factor VIII activity average: 135 units/bag. Also contains: Factor VIII, Factor XIII, Fibrinogen, Fibronectin and von Willebrand’s factor. Fibrinogen: 250 mg/bag. Action: Provides a source of coagulation Factor VIII, XIII, fibrinogen, VWF and Fibronectin. Indications: 1. Hypofibrinogenia or fibrinogen abnormality (dysfibrinogenemia). 2. Hemophilia A-Factor VIII deficiency (when factor concentrates are not available). 3. von Willebrand’s disease (second line of therapy behind DDAVP and/or Humate P). 4. Factor XIII deficiency. 5. Massive trauma. 6. DIC. 7. To prepare “fibrin glue”. Contraindications: 1. Do not use unless results of laboratory tests indicate specific coagulation defect for which this product would be beneficial. Cryoprecipitate should not be used to treat patients with deficiencies of factors other than Factor VIII, fibrinogen, von Willebrand’s Factor or Factor XIII. Side Effects & Hazards: 1. Infectious disease transmission 2. Air embolism 3. Febrile reactions 4. Allergic reactions 5. Hyperfibrinogenemia 6. Bacterial contamination Page 61 The Ohio State University Medical Center Transfusion Service On-Call Manual Dosage: 1. Hemophilia A: USUALLY NOT USED ANYMORE BECAUSE OF FACTOR CONCENTRATES. Varies on patient and clinical situation. If given, dosage can be calculated using the following formula: a. 40 ml/kg X Body Weight (kg) = PV (ml) b. Desired Factor VIII level (%) X Plasma Volume (ml) = 100 X 80 (minimum units Factor VIII per unit/bag) = number of bags of Cryoprecipitate AHF needed (Quick and Dirty: 0.5 bags/kg for Hemophilia A). To maintain hemostatic levels, repeat Factor VIII transfusions need to be given at 8-12 hour intervals; due to the 12-hour halflife of Factor VIII activity. c. 1 pool of CRYO will increase fibrinogen at least 30 mg/dl (except for local ARC pool at least 50mg/dl). d. neonate/children: 1 ml/kg or one single, random donor cryoprecipitate AHF/10 kg. 2. von Willebrand’s: a. Smaller amounts of Cryoprecipitated AHF will usually correct this deficiency, if DDAVP and Humate P are ineffective. (Type III-Humate P, Type I/IIa-DDAVP). b. Calculate Cryoprecipitated AHF dosage as for Factor VIII deficiency (roughly 1 bag/10 kg). 3. Fibrinogen >50<100 1 cryo pool. Fibrinogen <50 1 cryo pool, then re-evaluate. 4. Congenital Afibrinogenemia/Hypofibrinogenemia 4 bags/10 kg @ first. 2 bags/10 kg 24 hours later. Rate of Infusion: As quickly as possible (about 10 ml of diluted component per minute). Storage: 1. –18C or colder (1 year). 2. 20-24C (room temperature) after thawing. Shelf life: 1. 1 year in frozen state. 2. 4 hours after thawing or 4 hours after pooling. Administration: 1. Does not require ABO compatibility. If transfusing more than 350 ml per day, give ABO identical (crossmatch not required). 2. Thaw rapidly at 30-37C (up to 15 minutes). Page 62 The Ohio State University Medical Center Transfusion Service On-Call Manual 3. Maintain at room temperature until transfused; because, refrigeration may encourage reprecipitation of the concentrated Factor VIII. 4. Administer within 4 hours of thawing or 4 hours of pooling (whichever is shorter!!). 5. Do not refreeze after thawing. 6. Use blood component infusion set and syringe for IV push or blood component recipient set for IV drip (pooled). 7. 0.9 NaCl prn. CRYOPRECIPITATE, POOLED Volume: Approx. 100-140 ml (6 units per pool) Factor VIII activity: 480 units/pool Fibrinogen: >900mg (Supplier provides 1500mg/pool) Storage: 1. –18C or colder (1 year). 2. 20-24C (room temperature) after thawing. Shelf life: 1. 1 year in frozen state. 2. 4 hours after thawing. Rate of Infusion: IV drip as quickly as possible. Administration: 1. Does not require ABO compatibility unless transfusing more than 350 ml per day, then give ABO identical (crossmatch not required). 2. Thaw rapidly at 30-37C (up to 15 minutes). 3. Maintain at room temperature until transfused; because refrigeration may encourage reprecipitation of the concentrated Factor VIII. 4. Cryoprecipitated AHF, pooled, should be administered as soon as possible, but no longer than 4 hours after thawing. 5. Do not refreeze after thawing. 6. Y-type blood component recipient set for IV drip. 7. 0.9 NaCl prn. For additional information, refer to Circular of Information for the Use of Human Blood and Blood Components. GRANULOCYTES Medical approval is needed when ordering granulocytes. Page 63 The Ohio State University Medical Center Transfusion Service On-Call Manual Volume: 50 ml 3.2 X 109 WBC/bag Action: Fight infection. Need to monitor clinical response. Indications: The indications for the use of granulocyte transfusions are the presence of neutropenia and evidence of bacterial infection. 1. Absolute neutropenia (<500 PMN’s/l). 2. Documented sepsis, unresponsive to appropriate antibiotic therapy for 48 hours. The evidence of bacterial infection includes: 1. Positive culture from any internal body fluid. 2. Positive CIEP (Counter Immunoelectropheresis) or latex agglutination for bacterial antigens from body fluids. 3. Positive gram-stain from CSF or pleural fluid plus a clinical diagnosis of sepsis. Dosage: The efficacy of granulocyte transfusions has been correlated with the dosage given. It has been suggested that the ideal dosage would be equivalent to the total neutrophil pool. In the neonate, the total neutrophil pool is estimated to be 0.7-0.8 108 cells/kg. It seems reasonable that this should be taken as the minimal goal in neonatal granulocyte transfusion dosage. Adult: 1 pheresis product/day for 5-7 days Pediatric: 15 ml/kg body weight prepared as buffy coat from unit of whole blood. Storage: 20-24C Shelf life: 24 hours Administration: Blood component/recipient set. Depth-type microaggegate filter is prohibited. Leukodepletion filter is prohibited. Crossmatch required Prevention of Adverse Reactions: 1. Volume overload: This is prevented limiting the transfusion volume to 15 ml/kg and the transfusion rate to greater than 45 minutes. 2. GVHD: This is prevented by irradiating the products before transfusion. Page 64 The Ohio State University Medical Center Transfusion Service On-Call Manual 3. CMV Infection: This is prevented by procuring products from CMV-antibody negative donors when the infant is < 1500 g birth weight and has a seronegative mother. 4. Chills and fever, severe allergic reactions are common. diphenhydramine, steroids and or non-aspirin antipyretics. Premedicate with 5. Respiratory reactions with SOB, “white-out” on chest x-ray can occur. For additional information, refer to Circular of Information for the Use of Human Blood and Blood Components. ANTIHEMOPHILIC FACTOR (HUMAN) (Method M. Monoclonal Purified) Volume: Lyophilized Quantity of Factor VIII activity is stated on the bottle Action: Anti-Hemophilic Factor (human) is a plasma protein, which corrects the coagulation defect in patients with severe congenital Factor VIII deficiency (Hemophilia A). Indications: 1. Patients with moderate to severe congenital Factor VIII deficiency (Hemophilia A). 2. PATIENTS WITH LOW TITER FACTOR VIII INHIBITORS. 3. When used in patients with acquired Factor VIII inhibitors, the level of inhibitors should not increase >10 Bethesda units/ml. In these cases, the dosage should be controlled by frequent laboratory determinations of circulating AHF. CAUTIONS: 1. IDENTIFICATION OF THE CLOTTING DEFECT AS FACTOR VIII DEFICIENCY IS ESSENTIAL. 2. Do not use for treatment of von Willebrand’s disease (except Humate P). 3. Development of a positive direct antiglobulin test or hemolysis is possible due to the presence of anti-A or anti-B alloagglutinins in the concentrates. 4. Patient may form antibodies to mouse protein, inhibitors to clotting factors. 5. Patients may develop signs and/or symptoms of certain viral infections. 6. With high doses, marked elevations in fibrinogen. 7. Allergic reactions. 8. Venous thrombosis. Dose: 1. Units required = body weight (kg) X 0.4 units/kg X desired AHF increase (% of normal). Example: 70 kg x 0.4 units/kg X 50% = 1400 units Page 65 The Ohio State University Medical Center Transfusion Service On-Call Manual Expected AHF increase (% of normal) = Units administered Body weight (kg) X 0.4 units/kg Example: 1400 units 70 kg X 0.4 units/kg = 50% Half-life is 10-12 hours. 2. One unit of Factor VIII concentrate/kg will equate to a two percent increase in Factor VIII. Storage: 1. Recommended storage at 2-8C until reconstituted. 2. Bring to room temperature (22C) for reconstitution and administer within one hour. Do not refrigerate after reconstitution. 3. Do not freeze. Shelf life: 1. See expiration date on product. 2. Reconstituted Antihemophilic Factor (Human) should be used within one hour of reconstitution. Administration: 1. Intravenously. 2. See manufacturer’s instruction for reconstitution. Dispense through pharmacy. SERUM ALBUMIN, 5% SOLUTION Volume: 250 ml – 5% albumin 500 ml – 5% albumin Indications: 1. Plasmapheresis (Therapeutic Plasma Exchange) 2. Nephrotic syndrome: short-term use with diuretic treatment; acute severe peripheral or pulmonary edema. 3. Liver and pancreas transplantation: serum albumin <2.5g/dl, pulmonary wedge pressure <12mm Hg, Hct >30%. 4. Third line: a. Parocentesis Page 66 The Ohio State University Medical Center Transfusion Service On-Call Manual b. c. d. e. f. g. h. i. j. k. Hepatorenal syndrome Veno-occlusive disease (BMT) Hemorrhagic shock Non-hemorrhagic shock Burns Cerebral ischemia Hepatic resection Neonatal hypoalbuminemia Cardiac or vascular surgery Hemodialysis Contraindications: 1. In patients susceptible to fluid overload, albumin should be used with caution. 2. Albumin will not correct hypoproteinemia. 3. Should not be used for long-term therapy. Dosage: Depends upon clinical condition of patient, see package insert. Rate of Infusion: May be infused rapidly if necessary. Storage: Store at room temperature (22C). Shelf life: See expiration date on product. Administration: 1. Crossmatch is not required. 2. Blood component recipient set or may have administration set included. 3. Filter is recommended. Dispensed through pharmacy. For additional information, refer to The Ohio State University Medical Center, Clinical Practice Guidelines: Criteria for Use of Human Albumin on the intranet. IMMUNE SERUM GLOBULIN (ISG) Volume: 2 ml 10 ml Indications: 1. Provides passive prophylaxis of measles and Hepatitis A. 2. Replacement of gamma globulin in patients with hypo-agammaglobulinemia. Page 67 The Ohio State University Medical Center Transfusion Service On-Call Manual Contraindications: Individuals with a history of IgA deficiency or severe anaphylactic reactions to plasma should not be given ISG. Dosage: See manufacturer’s instructions. Storage: Recommended storage is at 2-8C. Shelf life: See expiration date on product. Administration: 1. See manufacturer’s instructions. 2. Intramuscular. 3. Divided doses may be given. Dispensed through pharmacy. IMMUNE GLOBULIN INTRAVENOUS (IGIV) Volume: Lyophilized Quantity stated on vial Indications: 1. Primary immunodeficiency states. 2. Prevention of bacterial infections associated with B-cell Chronic Lymphocytic Leukemia. 3. ITP. 4. Kawasaki Syndrome. 5. Primary agammaglobulinemia or secondary hypogammaglobulinemia. 6. Chronic inflammatory demyelinating polyneuropathy and other neurologic conditions such as GBS and myasthenia gravis. Dosage and Administration: See Manufacture’s instructions (IV administration only). Storage: 1. Store at a temperature not to exceed 25C. 2. Protect from freezing. Systemic reactions: 1. Urticaria, pruritus 2. Chills, fever, tremor Page 68 The Ohio State University Medical Center Transfusion Service On-Call Manual 3. Headache, flushing, malaise 4. Nausea, vomiting 5. Backpain, joint pains 6. Dyspnea, bronchospasm 7. Hypotension Dispensed through pharmacy. HEPATITIS B IMMUNE GLOBULIN (HBIG) Volume: 5 ml Action: 1. Hepatitis B Immune Globulin provides passive immunization for individuals exposed to Hepatitis B virus (HBV). Indications: 1. Persons who have had contact with Hepatitis B through needlestick, serum (blood) aspiration, oral, sexual, neonatal route. Contraindications: 1. Should be given with caution to patients with a history of prior systemic allergic reactions following administration of ISG. Dosage: Recommended dose is 0.06 ml per kilogram of body weight; the usual adult dose is 3 to 5 ml. Storage: Refrigerate at 2-8C. Shelf life: See expiration date on product. Administration: 1. Administer as soon as possible after exposure (preferable within 7 days. 2. Administer vaccine shortly after (same day or next day). Complete immunization schedule. 3. Administer intramuscularly. Dispense through pharmacy. Page 69 The Ohio State University Medical Center Transfusion Service On-Call Manual VARICELLA ZOSTER IMMUNE GLOBULIN (VZIG) Volume: Contains 125 units of antibody to varicella-zoster virus in a volume of 2.5 ml or less. Indication: 1. Provides passive immunization against varicella in immunosuppressed children who have been exposed. 2. Also used for prophylaxis in exposed (susceptible) pregnant women and organ transplant recipients. Contraindications: 1. Should not be administered to individuals having a history of severe reactions following administration of ISG. Dosage: Based on body weight. Kilograms Pounds Units # of Vials 0-10 0 -22 125 1 10.1 - 20 22.1 - 44 250 2 20.1 - 30 44.1 - 66 375 3 30.1 – 40 66.1 -88 500 4 over 40*** over 88 625 5 ***There is a single vial of 625 units for patients over 40 kilograms Storage: Refrigerate at 2-8C. Shelf life: See expiration date on product. Administration: 1. Administer the product by deep intramucular injection in the gluteal muscle. 2. Dispensed through Transfusion Service, requires Transfusion Medicine approval. PLASMA AND PLASMA DERIVATIVES AND THE TREATMENT OF DISEASE PPF (PLASMA PROTEIN FRACTION) (PLASMANATE = TRADE NAME) 1. Provides volume expansion and colloid replacement without the risk of hepatitis/HIV. 2. Used interchangeably with 5% albumin. 3. Dispensed from pharmacy. Page 70 The Ohio State University Medical Center Transfusion Service On-Call Manual 4. Cannot be infused rapidly (may cause hypotension). PROTHROMBIN COMPLEX 1. Contains the vitamin K dependent factors (II, VII, IX, X). 2. Used in cases of Hemophilia A with inhibitors and hemorrhage or invasive procedure. 3. Can be used for the rapid reversal of warfarin toxicity with or without bleeding. Much more potent and much less volume than FFP. 4. Dispensed by pharmacy. 5. Risk of thrombosis in liver disease. FACTOR IX CONCENTRATES 1. Used for replacement in Hemophilia B. 2. Monoclonal preparations contain no other factors except Factor IX. 3. During surgery, desired levels of Factor IX should be >50-75 U/dL. 4. Half of the initial dose in 12-24 hours. Two units of Factor IX concentrate/kg will equate to a one percent increase in Factor IX. 5. Dispensed by pharmacy. 6. Cannot be used for Factor VIII inhibitor patients since these only contain Factor IX as opposed to activated Prothrombin complexes, which contain enough vitamin K dependent factors to bypass Factor VIII’s step in coagulation cascade (especially in Hemophilia A patients with Factor VIII inhibitors. C1-ESTERASE INHIBITOR DEFICIENCY 1. Can cause angioneurotic edema. 2. Plasma (FFP) is a source of C1 esterase inhibitor. 3. New concentrates exist and are in the testing phase for both prophylactic and symptomatic use. ANTITHROMBIN III CONCENTRATES 1. Dispensed by pharmacy. 2. Approved for use in Antithrombin III deficiency (hereditary). 3. Not yet approved for acquired deficiency states (DIC, etc). Page 71 The Ohio State University Medical Center Transfusion Service On-Call Manual Procedures not listed either require no blood or are rarely performed. Service.Surgical/ Procedure I. DENTAL 1. All Procedures 0.0 II. EAR/NOSE/THROAT 2. Acoustic Tumor Excision T/S 3. Composite Resection 4 4. Esophagoscopy, Inj. Of Varices T/S 5. Ethmoidectomy, Complete T/S 6. Exploration of Maxilla T/S 7. G. Jugular/Skull Base Tumor Exc. 2 8. Glossectomy 2 9. Laryngectomy 2 10. Closure 2 11. Mandibulectomy 2 12. Maxillary Osteotomy T/S 13. Maxillectomy 4 14. Neck Dissection 2 15. Osteotomy T/S 16. Parotidectomy T/S 17. Radical Neck 1 III. GENERAL SURGERY 18. Abdominal Perineal Resect 1 19. Adrenalectomy T/S 20. Anterior Resection 1 21. Antrectomy T/S 22. Axillary Dissection T/S 23. Biliary Duct Exploration T/S 24. Bone Marrow Aspiration T/S 25. Chole Cutaneous Fistula Rep. T/S 26. Colon Resection T/S 27. Colostomy/Ileocolostomy T/S 28. Colostomy Closure T/S 29. Common Duct Exploration T/S 30. Debridement Choice 31. Dialysis AV Fistula/Shunt Rev. T/S 32. Duodenectomy T/S 33. Enteroenterostomy T/S 34. Flank Exploration Choice 35. Fundoplication T/S 36. Gastrectomy/Antrectomy T/S 37. Gastrojejunostomy T/S 38. Gastroplasty T/S 39. Gastrostomy T/S 40. Groin Exploration T/S 41. Hepatic Resection 4 42. Hepatico-Jejunostomy 2 43. Hernia, Hiatal Diaphragmatic T/S 44. Hernia, Repair Paraesophageal T/S 45. Hip Disarticulation 2 46. Ileostomy Closure T/S 47. Jejunostomy T/S 48. Jejunal Bypass/Take Down T/S Service.Surgical/ Procedure 49. Laparotomy Exploratory 50. Large Bowel Resection 51. Liver Transplant 52. Mastectomy 53. Pancreatectomy 54. Pancreatic Exploration 55. Pancreatic Pseudocyst 56. Protocolectomy 57. Rectal Bridge, Division 58. Rectal Carcinoma Excision 59. Rectal Prolapse Repair 60. Recto-Vaginal Fistula Repair 61. Renal/Pancreas TX Recipient 62. Renal Transplant Donor 63. Rib Resection 64. Sigmoid Colectomy 65. Sigmoid Resection 66. Skin Graft 67. Small Bowel Resection 68. Spincterotomy 69. Splenectomy 70. Staging Laparotomy 71. Stump Closure 72. Subhepatic Abscess Drain 73. Sympathectomy Abscess Drain 74. Thrombectomy 75. Thyroidectomy 76. Vagectomy/Vagotomy 77. Whipple IV. NEUROSURGERY 78. Aneurysm, Evaculation of Cran 79. Aneurysm, Clipping 80. Anterior Cervical Disc 81. AV Malformation 82. Brain Biopsy 83. Burr Holes 84. Carotid Ligation with Clamp 85. Cervical Disectomy 86. Cervical Rib Excision 87. Cordotomy 88. Cranioplasty 89. Crainiotomy 90. Crainiotomy, Tumor > 4 cm 91. Disc Cervical and Fusion 92. Disc Lumbar and Fusion 93. Excision Disc 94. Foraminotomy 95. Hypophysectomy 96. Laminectomy 97. Lumbar Decompression 98. Lumbar Peritoneal Shunt Page 72 T/S T/S Choice T/S 4 T/S 2 1 T/S 1 T/S T/S 4 T/S 2 T/S T/S Choice 1 T/S T/S T/S T/S 1 1 T/S T/S T/S 4 4 4 T/S 4 T/S T/S T/S T/S T/S T/S T/S T/S 4 T/S T/S T/S T/S T/S T/S T/S T/S The Ohio State University Medical Center Transfusion Service On-Call Manual Service.Surgical/ Procedure 99. Micro Dissection 100. Neuroma, Acoustic 101. Occipital Neurectomy 102. Pituitary Tumor 103. Shunts 104. Spinal Instrumentation 105. Subdural Hematoma 106. Supraclavicular Symphathecto 107. Transphenoidal Hypophysecto 108. Untether Spinal Cord V. OB/GYN 109. Abortion, Therapeutic 110. A/P Repair 111. C-Section 112. Cone Biopsy 113. Comual Reimplantation 114. Cystectomy 115. D&C 116. DS/Pre-Op Previa 117. Hysterectomy, Abd. Vag, TAH 118. Hysterectomy, Radical 119. Lymphadenectomy 120. Laparotomy/Cyst 121. Metroplasty 122. Mini-Lap Tubal 123. Myomectomy 124. Oophorectomy 125. Ovarian Cystectomy 126. Pelvic Exenteration 127. Rectocele 128. Rectovaginal Fistula 129. Salpingostomy 130. Tubal Reanastomosis 131. Tuboplasty 132. Uterine Myomectomy 133. Vaginoplasty 134. Vesicocele 135. Vulvectomy VI. ORTHOPEDIC 136. Anterior Spinal Release 137. Bankhart Procedure 138. Bil Grice Procedure 139. Bil Hip Abductor Rel Post Fus 140. Block Nail Tibia or Femur 141. Bone Graft 142. Closed Craig Needle Biopsy 143. Compression Screw, Hip 144. Corpectomy, Cervical 145. Costal Cartilage, Resection 146. Deltoid to Triceps Trans Bil 147. Dwyer Anterior Release 148. Enders Nail, Hip 149. Forage Procedure 150. Gaint Cell Tumor Excision T/S 2 T/S 2 T/S 4 1 T/S 2 1 T/S T/S T/S T/S T/S T/S T/S 2 T/S 1 1 T/S 2 T/S T/S T/S T/S 2 T/S T/S T/S T/S T/S T/S T/S T/S T/S 2 T/S T/S 4 1 1 T/S T/S 2 T/S 2 2 T/S 1 4 Service.Surgical/ Procedure 151. Girdlestone Procedure, Hip 152. Harrington Rod, Scoliosis 153. Harrington Rod, Fracture 154. Harrington Rod, Removal 155. Hip Arthroplasty 156. Hip Pinning 157. Hip, Replacement Pin Screws 158. Hip, Removal of Prosthesis 159. Hip Rev Long Stem Comp 160. Hockstad Rod, Insert 161. Hoffman Procedure, Other 162. IM Nail Femur with Circlage 163. IM Nail Tibia 164. Jewett Nail 165. Interspinous Wiring/Fusion 166. Kempf, Locke Nail, Tibia 167. K Nail 168. Knowles Pinning 169. Lugue Rods 170. Magnusen Repair Shoulder 171. Moore Prosthesis for Hip 172. Olecranon Fracture 173. Omega Hip Screw 174. Open Reduction, Arm, Leg 175. Open Reduction, Hip Anter. 176. Open Reduction, Hip Poster. 177. Open Reduction, Internal Fix 178. Osteotomy Hip, Pelvic, Femur 179. Osteotomy, Lumbar Spine 180. Osteotomy, Elbow, Knee 181. Osteotomy, Salter Innominate 182. Osteotomy, Tibia, Fibula 183. Pelvis, Internal Fix Sacroileac 184. Plate/Screws Femur, Insert 185. Post Fusion Harrington/LaRue 186. Resection 187. Removal Hardware, Other 188. Richards Compression 189. Richards Hip, Plate, Screw 190. Shoulder Reconstruction 191. Spinal Decompression 192. Spinal Fusion 193. Tendonesis, Bilateral 194. Total Elbow Relocating 195. Total Elbow Revision 196. Total Hip, Bilateral 197. Total Knee 198. Total Knee, Bilateral 199. Total Shoulder 200. Turco Procedure 201. Zickle Nail VII. PLASTIC 202. Abdomen Attenuation, Repair 203. Abdominoplasty Page 73 T/S 4 2 1 2 T/S T/S 2 3 2 2 2 T/S 2 2 T/S 2 T/S 4 T/S 1 T/S T/S T/S 4 2 T/S 2 T/S T/S 2 T/S 2 T/S 4 Choice T/S T/S T/S 2 2 2 1 T/S 2 2 1 2 1 T/S 4 1 T/S The Ohio State University Medical Center Transfusion Service On-Call Manual Service.Surgical/ Procedure 204. Cleft Palate 205. Debridement 206. Decubitus/Ulcer 207. Flap 208. Lipectomy, Abdominal 209. Palatal Fistual Closure 210. Reduction Mammoplasty 211. Skin Graft VIII. THORACIC 212. Aortic Valve 213. AV Fistula 214. CABG 215. Closure ASD 216. Commisurotomy 217. Decortication 218. Epicardial Lead Pacing Wire 219. Esophagogastrectomy 220. Esophago-Myotomy 221. Fundoplication 222. Heart Transplant 223. Injection Varices 224. Insertion Implantable Defib. 225. Lobectomy 226. Mediastinal Exploration 227. Mediastinoscopy 228. Mediastinotomy, Parasternal 229. Mitral Valve 230. Open Lung Biopsy 231. Open Pleural Biopsy 232. Open Rib Biopsy 233. Pectus Excavaturn 234. Pericardectomy 235. Pericardial Cyst. Exc. 236. Pericardial Window 237. Pharyngo-Esophageal Divert 238. Pleurectomy 239. Pnuemonectomy 12. Maxillary Osteotomy 240. Pulmonary Resection 241. Rib Resection 14. Neck Dissection 242. Scelerosing of Varcies 243. Sinus Venous Defect 244. Sternal Wiring 245. Sternotomy 246. Thoracotomy 247. Thoracoplasty 248. Thymectomy 249. Valvulotomy Pulmonic IX. UROLOGY 250. Adrenalectomy 251. Closure of Evisceration 252. Closure Vesico-Vaginal Fist. 255. Cyst Exploration T/S Choice Choice Choice 1 T/S T/S T/S 6 T/S 6 4 4 T/S 1 4 T/S T/S 6 T/S T/S 2 2 T/S 2 6 T/S T/S 1 T/S 4 2 T/S T/S T/S 2 T/S 2 T/S 2 T/S 4 2 2 2 T/S 4 4 Service.Surgical/ Procedure 256. Cystocele 257. Cystolithotomy 258. Diverticulectomy 259. Exploratory Celiotomy 260. Ileo Loop, Creat, Div, Rev. 261. Nephrectomy, Rad, Simple 262. Orchiectomy, Radical, Explor 263. Pelvic Lymph Node 264. Penectomy 265. Prostatectomy (TURP) 266. Prostatectomy, Open, Rad. 267. Retroperitoneal Lymph Node 268. Renal Artery Thrombectomy 269. Sphincter 270. Stricture 271. Suprapublic Cystotomy 272. TUR-BT 273. Ureteral Implant 274. Ureterectomy 275. Ureterolysis 276. Urethral Fistula Repair 277. Urethral Suspen, Tapering 278. Urethropexy/plasty 279. Vesicopexy, Anterior, Needle 280. Vesicostomy X. VASCULAR 281. Amputation A/K 282. Aneurysm, Femoral 283. Aneurysm, Popliteal, Brachial 284. Aneurysm, Thor/Abdominal 285. Aorto Bifemoral Bypass 286. Carotid Bypass 287. Embolectomy 288. Endarterectomy, Renal Artery 289. Endarterectomy, Carotid 290. Endarterectomy, Aortic Ileac 291. Fem-Fem Graft 292. Femoral-Popriteal Graft 293. Femoral-Popriteal Thrombect. 294. Fem-Tib Bypass Graft 295. Popliteal Aneurysm 296. Portacaval Shunt 297. Renal Artery Reconst/Bypass 298. Spleno-Renal Shunt 299. Subclavian Bypass 300. Vena Cava Umbrella Replace XI. OTHER 301. Specify On Order 2 T/S T/S T/S Page 74 T/S T/S T/S T/S T/S 2 T/S T/S T/S T/S 2 2 2 T/S T/S T/S T/S T/S T/S 2 T/S T/S T/S T/S T/S T/S 2 1 4 4 2 T/S 4 2 4 1 1 T/S 1 1 4 4 4 1 2 Choice The Ohio State University Medical Center Transfusion Service On-Call Manual TRANSFUSION GUIDELINES TRANSFUSIONS IN ADULTS Definitions of Recommendation Levels (The quality of evidence determines the strength of the guideline.) Quality of Evidence Level of Evidence Definition I Good Evidence obtained from at least one properly randomized controlled trial or from well-designed cohort or case-control analytic studies, preferably from more than one center or research group, or national consensus panel recommendations based on controlled, randomized studies. II Fair Evidence obtained from multiple time series with or without intervention, or national consensus panel recommendations based on uncontrolled studies with positive outcomes or based on studies showing dramatic effects of an intervention. III Poor Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees. Strength Strength of Recommendation Definition A It is advised that this be done. . . in a periodic health examination, or a diagnostic maneuver should be done, or treatment of a medical problem should be given. There is good evidence (level I) to support the recommendation. B This may be done . . . in a periodic health examination, or a diagnostic maneuver may be done, or treatment of a medical problem may be given. B1 It should be done in most cases. There is fair evidence (level II) to support the recommendation. B2 It should NOT be done in most cases. There is poor evidence (level III) regarding the recommendation, which may be made on other grounds. C It is advised that this NOT be done . . . in a periodic health examination, or a diagnostic maneuver should not be done, or treatment of a medical problem should not be given. There is good evidence (level I) to support the recommendation that this not be done. Note: Practice guidelines are not standards that are meant to be applied rigidly and followed in virtually all cases. Patient choice and physician judgment must remain central to the selection of diagnostic tests and therapy. Practice guidelines should be helpful to physicians and patients alike in making their decisions. At the Ohio State University Medical Center, clinical practice guidelines are reviewed annually to incorporate new evidence. Page 75 The Ohio State University Medical Center Transfusion Service On-Call Manual OBJECTIVE To define the appropriate use of blood components for transfusion therapy. GUIDELINE STATEMENT (“B1” Recommendation: This should be done in most cases. There is fair evidence [Level II] to support the recommendation.) Patients should be evaluated for blood component therapy by clinical and laboratory assessment. The appropriate blood component is selected and given in the appropriate dose. KEY WORDS blood, blood products, transfusion BACKGROUND RED BLOOD CELLS The main purpose of red blood cell transfusion is to increase oxygen-carrying capacity. However, the critical hemoglobin level requiring transfusion is unclear. Animal data indicate that hemoglobin levels of 3-5 g/dl are critical; below those levels, decompensation for the anemia occurs. For healthy humans, the hemoglobin level for decompensation is similar at something less than 5 g/dl. One study deliberately bled patients and volunteers to hemoglobin levels < 5.0 g/dl without clinical sequelae. 1 Several randomized clinical trials have shown no differences in outcome whether the transfusion triggers were high or low. One recent large random controlled trial (RCT) showed that mortality was less for those critically ill patients who received red blood cells for hemoglobin levels < 7.0 g/dl than for those transfused to maintain the hemoglobin > 10 g/dl.2 Patients with cardiac disease showed no differences in outcomes in this study. Another study of elderly patients > 60 years of age showed that transfusion had no effect on mortality as long as the hemoglobin level was > 8 g/dl.3 Two studies of Jehovah’s Witnesses showed that mortality did not increase for hemoglobin < 5 g/dl, even for cardiovascular patients.4, 5 One patient with postoperative hemoglobin of 2.7 g./dl survived. Recent published guidelines have cautioned against a uniform hemoglobin level as an indication for red blood cell transfusion. The American Society of Anesthesiologists Task Force on Blood Component Therapy6 as well as the Red Blood Cell Administration Practice Guideline Development Task Force of the College of American Pathologists 7 recommend that transfusion is rarely indicated when the hemoglobin level is > 10 g/dl, and almost always indicated when the hemoglobin level is < 6 g/dl. If the hemoglobin level is between 6 and 10 g/dl, transfusions should be “based on the patient’s risk for complication of inadequate Page 76 The Ohio State University Medical Center Transfusion Service On-Call Manual oxygenation.” 6, 7 In acute hemorrhage, the hemoglobin level may not reflect the severity of hemorrhage nor the true amount of blood loss. Other considerations to limit or avoid transfusions include careful surgical hemostasis, limiting blood loss for laboratory assays, use of specific pharmacological agents in chronic anemia (e.g., vitamins, erythropoietin), reducing risk of hemorrhage caused by many types of drugs (e.g., aspirin, warfarin, heparin, low molecular weight heparin), and correcting any existing coagulopathy. PLATELETS The purpose of platelet transfusion is to treat clinically significant thrombocytopenia and treat hemorrhage caused by thrombocytopenia or platelet functional disorders. In the absence of clinically significant hemorrhage, the customary platelet count triggering platelet transfusion has been 20,000/μl. However, several authors have evidence for a lower transfusion trigger, such as 5,000/μl or 10,000/μl in stable patients. 8,9,10,11 Patients with concomitant conditions, such as sepsis, antibiotic therapy, and other abnormalities of hemostasis, may require higher transfusion triggers. For hemorrhage or invasive procedures, the recommended level is 50,000/μl.12,13 Two platelet preparations are available: platelet concentrates prepared from whole blood, and platelets collected by apheresis. For most blood suppliers, 4-6 units (bags) of platelet concentrates are equal to one plateletpheresis. Platelet donors may be selected for their CMV status, HLA type, or platelet crossmatch compatibility, as well as ABO and Rh types. Patients who have poor posttransfusion platelet recovery and survival present challenges for platelet therapy.14,15 Sepsis, hypersplenism, fever, DIC, amphotericin, hemorrhage, ABO mismatch, and alloimmunization affect platelet recovery and survival. The cause of poor platelet recovery and survival in most patients is nonimmune. A trial of ABO matched and fresh (less than 3 days old) platelet products for transfusion can be worthwhile. For alloimmunized patients, antibodies may be HLA and/or platelet specific. HLA matching requires HLA typing for the patient and donor, and collection, processing, and delivery of the plateletpheresis product. On the other hand, platelet crossmatching can be performed on platelet products in inventory. Because of this, platelet crossmatched products are more readily available. FRESH FROZEN PLASMA Fresh frozen plasma (FFP) is transfused to correct hemostatic abnormalities. Generally, hemostasis is adequate when coagulation factors are at least 20-30% of normal and fibrinogen is greater than 75 mg/dl. Bleeding is generally associated with PT and/or PTT values greater than 1.5 to 1.8 times normal during surgery or massive transfusion.16,17 However, abnormal PT and PTT values up to 2.0 times normal are poor predictors of bleeding associated with minor invasive procedures such as paracentesis, thoracentesis, percutaneous liver biopsy, and central venous catheterizations.18,19,20 Page 77 The Ohio State University Medical Center Transfusion Service On-Call Manual In addition, expert opinion does not support the use of FFP to reverse warfarin effect associated with bleeding or before invasive procedures if the INR is < 1.8. 21,22 For surgery, expert opinion supports holding one or more doses of warfarin or administering low-dose vitamin K (2-4 mg orally) and using postoperative prophylaxis with low molecular weight heparin and warfarin.23 Generally, massive transfusion is not accompanied by excessive bleeding until more than one blood volume is replaced. Correcting thrombocytopenia has been shown to be of greater importance in excessive microvascular bleeding than correcting PT and PTT prolongation.24 For patients receiving heparin, protamine sulfate, rather than FFP, should be used to correct prolonged PTT. CRYOPRECIPITATE-POOR PLASMA Cryoprecipitate-poor plasma (also called cryo-poor plasma) is the supernatant remaining after separation of cryoprecipitate. The high-molecular-weight von Willebrand factor is removed in this process. As high-molecular-weight von Willebrand factor is present in hemolytic uremic syndrome and thrombotic thrombocytopenia purpura, cryo-poor plasma and solvent detergent (SD)-treated plasma (see below) are preferred for therapy of these diseases.25 Cryoprecipitate-poor plasma may be used as a direct infusion in patients with TTP/HUS or as the replacement fluid during therapeutic plasma exchange (plasmapheresis.) SOLVENT-DETERGENT (SD)-TREATED PLASMA Solvent-detergent-treated plasma is prepared from large pools of fresh frozen plasma, cleared of the solvent and detergent, filtered, and transferred in 200 ml aliquots to bags, which are again frozen. The solvent-detergent treatment is effective against lipid enveloped viruses such as HIV and hepatitis B and C in the donor plasma. Disadvantages are the smaller volume compared to FFP (200 ml vs. 250-300 ml), possible decreased hemostatic effectiveness, and the higher cost per bag. CRYOPRECIPITATE Cryoprecipitate is rich in factor VIII, fibrinogen, von Willebrand factor, and factor XIII. However, factor concentrates, treated to be virally safe, are available for treatment of factor VIII deficiency and von Willebrand disease. Factor XIII is a rare deficiency. Therefore, cryoprecipitate is used to treat hypofibrinogenemia and as a source for fibrin glue. There is little scientific evidence about the effectiveness of cryoprecipitate in the treatment of hypofibrinogenemia; however, expert opinion agrees that cryoprecipitate is likely to be effective when used to correct fibrinogen levels < 80 mg/dl.6 Page 78 The Ohio State University Medical Center Transfusion Service On-Call Manual SPECIAL PRODUCTS Leukocyte-Reduced Red Blood Cells and Platelets Leukocyte-reduced blood is used to prevent febrile non-hemolytic transfusion reactions in patients with repeated or severe febrile reactions. Several studies have shown that leukocyte reduction with fourth-generation filters (those in use currently) are effective in reducing transmission of cytomegalovirus (CMV) by transfusion in premature neonates and patients with bone marrow or solid organ transplants. 26,27,28 Leukocyte depletion of platelet transfusions has been shown to reduce the incidence of human leukocyte antigen (HLA) alloimmunization in patients who are anticipated to receive repeated platelet transfusions. Similarly, leukocyte depletion of red blood cells may reduce HLA alloimmunization in patients waiting for organ transplantation. Many studies have examined the effect of transfusion on cancer recurrence, metastasis, and mortality. A pooled analysis of 20 studies showed that if an adverse effect exists, it has to be small.29 Posttransfusion infections have been studied, with rates of infection about 20-30% with allogeneic transfusion versus about 0-10% with autologous transfusion.30 A large prospective trial did not show a reduction in length of stay for cardiac surgery patients receiving leukocyte-reduced blood. Another concern has been new variant Creutzfeldt-Jacob disease (nvCJD). Prions have been found in human lymphocytes, granulocytes, platelets, and plasma. However, no evidence has been found that CJD has been transmitted by blood transfusion. Cytomegalovirus (CMV) Seronegative Red Blood Cells and Platelets Donor CMV status is important for premature infants, seronegative recipients of solid organ and autologous bone marrow transplants, and all allogeneic bone marrow transplant recipients. CMV disease in these patients can be severe and life threatening. Only about 40% of the donor population in this area is CMV seronegative. Therefore, CMV seronegative RBC and platelets are in short supply. Leukocyte-reduced blood products are equally effective as seronegative blood products in preventing CMV transmission. Gamma Irradiation Transfusions-associated graft-versus-host disease (TA-GVHD) is caused by donor lymphocytes proliferating in the recipient. The risk of the disease is increased for patients who are immunosuppressed (bone marrow transplant, Hodgkin’s disease) and those with immature immune systems (low birth weight neonates). However TA-GVHD has also been reported in immunocompetent patients, especially those receiving fresh blood or blood from blood relatives. TA-GVHD can be prevented by gamma irradiation of cellular blood products. ADVERSE REACTIONS The most common adverse transfusion reactions are febrile reactions, urticarial reactions, and volume overload. Febrile reactions can be treated and prevented by Page 79 The Ohio State University Medical Center Transfusion Service On-Call Manual antipyretics such as acetaminophen; urticarial reactions respond to diphenhydramine. Rarer are the hemolytic reactions. The most common symptom of hemolytic transfusion reaction is fever; therefore, fever in a patient receiving RBC transfusion should be investigated to rule out a hemolytic reaction. Another rare, but serious, reaction is transfusion-related acute lung injury (TRALI), caused by aggregation of leukocytes in the pulmonary vasculature. Treatment is respiratory support. Most cases resolve in a few days. Many diseases can be transmitted by blood; however, transmission is generally rare. The estimated risk of transmission of HIV is < 1:600,000. RECOMMENDATIONS Red Blood Cell Transfusion Hemoglobin Recommendation Level of Rec. > 10 g/dL 6.0-10.0 g/dL < 6.0 g/dL Acute blood loss > 20% B.V. RBC transfusion rarely needed RBC needed only for symptomatic patients RBC transfusion generally needed Fluid resuscitation; RBC transfusion B1 B1 B1 B1 Platelets Acute Hemorrhage or Invasive Procedure Level of Rec. Platelet count < 50,000/ul Platelet dysfunction unresponsive to other modalities (e.g., DDAVP, dialysis) B1 B1 Prophylaxis Level of Rec. Platelet count < 10,000/μl Platelet count < 20,000/μl Platelet count < 20,000/μl in next 24 hours Stable patient High-risk patient Decreasing platelet count B1 B1 B1 Fresh Frozen Plasma (FFP) Active Bleeding or Invasive Procedure Level of Rec. INR > 1.8 (PT > 18 sec.) APTT > 60 sec. (Use protamine sulfate to correct PTT prolongation due to heparin.) Coagulation factor assay < 25% Urgent reversal of warfarin (INR > 1.8) (hold dose or low-dose vitamin K preferred) Multiple coagulation factor deficiency (severe liver disease, DIC, vitamin K depletion) B1 B1 B1 B2 B1 Other Level of Rec. Plasma exchange for TTP/HUS (second line) B1 RECOMMENDATIONS (CONTINUED) Cryoprecipitate-Poor Plasma Plasma exchange for TTP/HUS Level of Rec. B1 Cryoprecipitate Page 80 The Ohio State University Medical Center Transfusion Service On-Call Manual Fibrinogen Condition Level of Rec. < 50 mg/dL < 100 mg/dL Stable patient Acute hemorrhage or invasive procedure B1 B1 von Willebrand’s disease (second line) Fibrin surgical adhesive B1 B1 Leukocyte-Reduced Red Blood Cells and Platelets Leukocyte-reduced blood transfusion is recommended for: Patients with severe or repeated febrile non-hemolytic transfusion reactions. Patients for whom CMV seronegative red blood cells and platelets are indicated. Patients who are anticipated to receive repeated platelet transfusions (to reduce HLA alloimmunization). Cytomegalovirus (CMV) Seronegative Red Blood Cells and Platelets CMV seronegative red blood cells and platelets are recommended for: Premature infants, seronegative recipients of solid organ and autologous bone marrow transplants, and all allogeneic bone marrow transplant recipients Gamma Irradiation Gamma irradiation of cellular blood products is recommended for prevention of transfusion-associated graft versus host disease in: Patients who are immunosuppressed (bone marrow transplant, Hodgkin’s disease) Patients with immature immune systems (low birth weight neonates) Some immunocompetent patients, especially those receiving fresh blood or blood from blood relatives Page 81 Level of Rec. B1 B1 B1 Level of Rec. B1 Level of Rec. B1 B1 B1 The Ohio State University Medical Center Transfusion Service On-Call Manual RECOMMENDATIONS (CONTINUED) Adverse Reactions Febrile reactions to transfusion - can be treated and prevented by antipyretics such as acetaminophen Urticarial reactions - can be treated with diphenhydramine Fever in a patient receiving RBC transfusion - should be investigated to rule out hemolytic reaction Transfusion-related acute lung injury (TRALI) - treated with respiratory support Page 82 Level of Rec. B1 B1 B1 B1 The Ohio State University Medical Center Transfusion Service On-Call Manual ADAPTED BY Melanie Kennedy, MD; Department of Pathology; Division of Transfusion Medicine. APPROVED BY The Clinical Practice Guideline Policy Group, June 21, 2000. The Leadership Council for Clinical Value Enhancement, ____________________. COPYRIGHT © 2000. The Ohio State University. No part of this publication may be reproduced in any form without permission in writing from The Ohio State University. Page 83 The Ohio State University Medical Center Transfusion Service On-Call Manual REFERENCES - TRANSFUSION GUIDELINES 1. Weiskopf RB, Viele MK, Feiner J, et al. Human Cardiovascular and Metabolic Response to Acute, Severe Isovolemic Anemia. JAMA 279:3:217-221, 1998. 2. Hebert PC, Wells G, Blajchman MA, et al. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N Engl J Med 340:6:409-417, 1999. 3. Carson JL, Duff A, Berlin JA, et al. Perioperative Blood Transfusion and Postoperative Mortality. JAMA 279:3:199-205, 1998. 4. Spence RK, Alexander JB, DelRossi AJ, et al. Transfusion Guidelines for Cardiovascular Surgery: Lessons Learned from Operations in Jehovah’s Witness. J Vasc Surg 16:825-31, 1992. 5. Viele MK, Weiskopf RB. What can we learn about the need for transfusion from the patients who refuse blood? The experience with Jehovah’s Witnesses. Transfusion 34:396-401, 1994. 6. American Society of Anesthesiologists Task Force on Blood Component Therapy. guidelines for blood component therapy. Anesthesiology 84:732-747, 1996. Practice 7. Simon TL, Alverson DC, AuBuchon J, et al. Practice Parameter for the Use of Red Blood Cell Transfusions. Arch Pathol Lab Med Vol. 122:130-138, 1998. 8. Rebulla P, Finazzi G, Marangoni F, et al. The Threshold for Prophylactic Platelet Transfusions in Adults with Acute Myeloid Leukemia. N Engl J Med 337:26:1871-1875, 1997. 9. Heckman KD, Weiner GJ, Davis CS, et al. Randomized Study of Prophylactic Platelet Transfusion Threshold During Induction Therapy for Adult Acute Leukemia: 10,000/L Versus 20,000/L. Am J Clin Oncol 15:3:1143-1149, 1997. 10. Sagmeister M, Oec L, Gmür J. A Restrictive Platelet Transfusion Policy Allowing Long-Term Support of Outpatients with Severe Aplastic Anemia. Blood 93:9:3124-3126, 1999. 11. Wandt H, Markus F, Ehninger G, et al. Safety and Cost-Effectiveness of a 10 x 109/L Trigger for Prophylactic Platelet Transfusions Compared with the Traditional 20 x 10 9/L Trigger: A Prospective Comparative Trial in 105 Patients with Acute Myeloid Leukemia. Blood 91;10:3601-3606, 1998. 12. Royal College of Physicians of Edinburgh. Consensus Conference on Platelet Transfusion. British J Cancer 78(3):290-291, 1998. 13. Office of Medical Applications of Research. Platelet Transfusion Therapy. JAMA 257:13:1777-1780, April 1987. 14. McFarland JG, Anderson AJ, Slichter SJ. Factors Influencing the Transfusion Response to HLASelected Apheresis Donor Platelets in Patients Refractory to Random Platelet Concentrates. British J Haematol 73:380-386, 1989. 15. Slichter SJ. Algorithm for Managing the Platelet Refractory Patient. J Clin Apheresis 12:4-9, 1997. 16. Domen RE, Kennedy MS, Jones LL, Senhauser DA. Hemostatic Imbalances Produced by Plasma Exchange. Transfusion 24:336-339, 1984. 17. Murray DJ, Pennell BJ, Weinstein SL, Olson JD. Packed Red Cells in Acute Blood Loss: Dilutional Coagulopathy as a Cause of Surgical Bleeding. Anes Analg 80:336-342, 1995. 18. McVay PA, Toy PTCY. Lack of Increased Bleeding After Paracentesis and Thoracentesis in Patients with Mild Coagulation Abnormalities. Transfusion 31:164-171, 1991. ( 19. Ewe K. Bleeding After Liver Biopsy Does Not Correlate with Indices of Peripheral Coagulation. Dig Dis Sci 26:5:388-393, 1981. 20. Foster PF, Moore LR, Sankary HN, et al. Coagulopathy. Arch Surg 127:273-275, 1992. Central Venous Catheterization in Patients with (Continued on next page) Page 84 The Ohio State University Medical Center Transfusion Service On-Call Manual REFERENCES - TRANSFUSION GUIDELINES (CONTINUED) 21. Practice Parameter – College of American Pathologists. Practice Parameter for the Use of FreshFrozen Plasma, Cryoprecipitate, and Platelets. JAMA 271:10:777-781, 1994. 22. Plasma – Consensus Conference. Fresh-Frozen Plasma. JAMA 253:4:551-553, Jan 1985. 23. Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness and optimal therapeutic range. Chest 114;445S-469S, 1998. 24. Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg 190:91-99, 1979. 25. Owens MR, Sweeney JD, Tahhan RH, Fortkolt P. Influence of type of Exchange Fluid on Survival in Therapeutic Apheresis for Thrombotic Thrombocytopenic Purpura. J Clin Apheresis 10:178-182, 1995. 26. Bowden RA, Slichter SJ, Sayers M, et al. A Comparison of Filtered Leukocyte-Reduced and Cytomegalovirus (CMV) Seronegative Blood Products for the Prevention of Transfusion-Associated CMV Infection after Marrow Transplant. Blood 86:3598-3603, 1995. 27. De Graan-Hentzen YCE, Gratama JW, Mudde GC, Verdonck LF. Prevention of Primary Cytomegalovirus Infection in Patients with Hematologic Malignancies by Intensive White Cell Depletion of Blood Products. Transfusion 29:757-760, 1989. 28. Van Prooijen HC, Visser JJ, Van Oostendorp WR, et al. Prevention of Primary TransfusionAssociated Cytomegalovirus Infection in Bone Marrow Transplant Recipients by the Removal of White Cells from Blood Components with High-Affinity Filters. British J Haematology 87:144-47, 1994. 29. Vamvakas E, Moore SB. Perioperative Blood Transfusion and Colorectal Cancer Occurrence: A Qualitative Statistical Overview and Meta-analysis. Transfusion 33:9:754-765, 1993. 30. Blumberg N, Triulzi DJ, Heal JM. Transfusion-Induced Immunomodulation and Its Clinical Consequences. Transfusion Med Rev IV:4 (Suppl 1):24-35, 1990. Page 85 The Ohio State University Medical Center Transfusion Service On-Call Manual REFERENCES – ON CALL MANUAL 1. Klein HG. Standards for Blood Bank and Transfusion Services. 17th ed. Bethesda, MD: American Assocation of Blood Banks, 1996. 2. National Archives and Records Administration. Code of Federal Regulations (Food and Drugs Title 21. Washington, DC: Office of the Federal Register National Archives and Record Administration, Parts 211, 600, 610, 640, 1994. 3. Walker RH. Technical Manual. 11th ed. Bethesda, MD: American Association of Blood Banks, 1993. 4. Vengelen-Tyler, V. Technical Manual. 12th ed. Bethesda, MD: American Association of Blood Banks, 1996. 5. Jones FS. Accreditation Requirements Manual. 5th ed. Bethesda, MD: American Association of Blood Banks, 1994. 6. Circular of Information for the Use of Human Blood and Blood Components. The American Red Cross, Council of Community Blood Centers and American Association of Blood Banks, 1996. 7. General Laboratory Procedure Manual. Service, 1996. The Ohio State University Medical Center, Transfusion 8. Reference/Prenatal Procedure Manual. Service, 1996. The Ohio State University Medical Center, Transfusion 9. Hospital Manual. The American Red Cross, Central Ohio Region Blood Services, 1995. 10. Henry JB. Clinical Diagnosis and Management by Laboratory Methods, 19th ed. W.B. Saunders – Philadelphia 1996. 11. Rossi EC, et al. Principles of Transfusion Medicine. Williams and Wilkins: Baltimore, MD: 2nd ed, 1996. 12. Winthrobe MN. Therapy of the Hereditary Coagulation Disorders. Philadelphia: Lea and Febiger 1190, 1979. 13. Westphal RG, et al. Arlington, VA, 1992. In: Clinical Hematology, Transfusion Management of Some Common Heritable Disorders. AABB, 14. Chamberland M, Khnnaz R. Emergency Issues in Blood Safety, Inf Dis Clin NAM 12(1):217-29, 1998. 15. Petz LD. Drug-induced Hemolytic Anemia. Transfusion Med Rev 7(4):242-54, 1993. 16. Jefferies LC. Transfusion Therapy in Drug-Induced Hemolytic Anemia. Hen OncClin NAM 8(6):1087194. 17. Physicians’ Desk Reference, 52nd Edition. Medical Economics – New Jersey, 1998. The authors wish to acknowledge the other individuals who contributed to this manual: Mary E. Wissel, M.D., Richard Morgan, M.D., Randy Sosolik, M.D., Gina Fino, M.D., Aamir Ehsan, M.D., Carmen Julius, M.D., Larry Lasky, M.D., and Frank Counts, MLT(ASCP)SBB. C:\Documents and Settings\huds03\Desktop\xMedmanual2002.doc Page 86