Overall Analysis of Vanillyl-Alcohol Oxidase and
advertisement

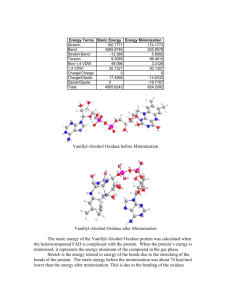
Jeff Hamilton Protein Modeling of the Heterocompound Flavin Adenine Dinulecotide and of the Protein VanillylAlcohol Oxidase Introduction The dimeric protein Vanillyl-Alcohol Oxidase from Penicillium simplicissimum catalyzes the oxidation of some 4-hydroxybenzyl alcohols. For example, VanillylAlcohol Oxidase catalyzes the oxidation of vanillyl alcohol to produce canillin and hydrogen peroxide. It has the heterocompound Flavin adenine dinucleotide attached to the protein and it aids in catalyzing the reaction. Protein: Vanillyl-Alcohol Oxidase Protein number: 1AHZ Heterocompound: Flavin Adenine Dinucleotide Het code: FAD Part I The heterocompound protein Flavin Adenine Dinucleotide and the VanillylAlcohol Oxidase which complexes with FAD was selected. The file of the complex was downloaded from the RCSB website to the program DS Visualizer. The heterocompound was extracted from the complex and the geometry for FAD was corrected. Figure 1: Heterocompound FAD Part II In DS Visualizer, the protein was shown as a solid ribbon format. The heterocompound is within the protein and it is displayed in a ball and stick format with the different elements displayed in different colors. 1 Jeff Hamilton Figure 2: The protein in solid ribbon form with the Heteroatom Part III The PDB file of the heterocompound was imported into Chem3D Ultra. The steric energy of the heterocompound was calculated. Then the program was used to perform a energy minization of the heteroatom (Figure 4). Table 1: Steric Energy and Energy Minimization Calculations of the FAD Energy Terms Steric Energy Energy Minimization Stretch: 100.7771 174.1773 Bend: 4365.8746 525.8078 Stretch-Bend: -12.389 5.6692 Torsion: 9.3069 98.4615 Non-1,4 VDW: 49.096 3.3126 1,4 VDW: 35.7321 50.1307 Charge/Charge: 0 0 Charge/Dipole: 17.4266 -14.6333 Dipole/Dipole: 0 -18.7167 Total: 4565.8243 824.2092 Figure 3: FAD before Minimization 2 Jeff Hamilton Figure 4: FAD after Minimization The steric energy of Flavin Adenine Dinucleotide (FAD) was calculated. When FAD’s energy was minimized, it represents the energy minimum of the compound in the gas phase. Stretch is the energy related to energy of the bonds due to the stretching of the bonds of the protein. The steric energy before the minimization was about 74 kcal/mol lower than the energy after minimization. This is due to the bending of FAD around the P-O bonds of the phosphate groups. Bend is the energy related to the bond angles that keep the bonds from the most stable angle or conformation. The Bend for the gas phase heterocompound was about 3840 kcal/mol less then the bend for the heterocompound before minimization. This is due to the high van der Waals forces between electron clouds in the protein before minimization which causes the bend energy to be high. The bend contributed the most energy to the steric energy of both compounds. The stretchbend is the energy that involves two bonds that form a bond angle and when that bond is strained. The heterocompound has about 17 kcal/mol less stretch-bend energy then the heterocompound in the gas phase. This is due to the bending of the energy minimized oxidase around the P-O bonds. The stretch bend contributed little to the overall steric energy of both compounds. The torsion is the energy from deviating dihedral angles from their most favorable values. The torsion energy for the heterocompound was about 88 kcal/mol less then the heterocompound in the gas phase. This shows that the heterocompound before minimization has more stability from angles that are closer to their optimal dihedral angle. The non-1,4 VDW is the energy related to the repulsive forces of the electron clouds between atoms that are farther then three atoms apart. The FAD in the gas phase had about 46 kcal/mol less non-1,4 VDW steric energy then the FAD from the heterocomplexed compound. This is due to the bending of FAD in the gas phase to produce more favorable interactions. The 1,4 VDW is the energy from the repulsion of the electron clouds of the atoms that are two atoms apart. The 1,4 VDW of the FAD in the gas phase was about 15 kcal/mol higher then the FAD from the hetero-complexed protein since the bending caused the oxygens around the phosphate groups to be closer together, causing more repulsions as the molecule bend to its energy minimized conformation. 3 Jeff Hamilton The charge/charge is the sum of the pairs of electrostatic interactions between charged atoms. The charge/charge interactions in both of the phases of the FAD turned out to be zero. The electrostatic interactions between the two compounds did not change from the minimization. The charge/dipole is the electrostatic energy from the interaction of a charged group and of a group with a dipole. The charge/dipole of the FAD in the gas phase is about 31 kcal/mol lower then the FAD before minimization. The FAD in the gas phase had more favorable dipole/charge interactions then the FAD from the heterocomplexed compound. Dipole/dipole is the energy of the interactions between two groups with dipoles. The energy minimized compound had a dipole/dipole that was 18 kcal/mol lower since it was in a conformation that allowed more dipole interactions between the lone pairs of the oxygens and the hydrogens bonded to carbon. The total energy is the sum of all the steric energies of the compound. The FAD in the gas phase is much more stable then the FAD from the hetero-complexed oxidase since the FAD in the gas phase had about 5740 kcal/mol less of steric energy within the compound. Part IV On Chem3D Ultra, an overlay of the energy minimized heterocompound and the heterocompound from the hetero-complexed protein was performed. Figure 5: Overlay of the Two FAD compounds Part V The amino acid sequence of the protein was obtained from the wiring diagram in the PDBsum site (Figure 6). The red dots indicate all of the amino acids of the protein that interact with the heterocompound and the amino acid. The amino acids in red boxes are in the active site of the protein. The ligplot of the protein was obtained from the PDBsum site and it shows the amino acids of the protein that interact with the heterocompound (Figure 7). The amino acids that interact with FAD where highlighted in yellow in DS Visualizer. 4 Jeff Hamilton Figure 6: Wiring Diagram of the Protein 5 Jeff Hamilton Figure 7: Ligplot of the Heterocompound FAD Figure 8: Amino acids that interact with FAD in DS Visualizer (amino acids that did not interact with FAD where not shown) The LIGPLOT shows the interactions between the amino actions of the protein such as the hydrogen bonds (dotted green lines). The nitrogen of Arg 504 hydrogen bonds with the lone electron pairs of the oxygen which is a part of a phosphate group. The “eyelashes” are the hydrophobic interactions or other non-hydrogen bonding interactions that occur between the amino acids and FAD. For example, the hydrophobic amino acid Trp 413 interacts with the non-polar methylene group of FAD. Also the oxygen of Glu 260 interacts with the lone pairs of electrons on the nitrogen of the adenine of the FAD molecule. 6 Jeff Hamilton Figure 9: Flavin adenine Dinucleotide (ball and stick) within the Vanillyl-Alcohol Oxidase (solid ribbon) Figure 10: The amino acids that interact with FAD are shown in a pale yellow 7 Jeff Hamilton Figure 11: The hetero-complex compound showing the interactions between FAD and the amino acid side chains. Part VI The amino acids that interact with FAD were identified and analyzed in Table 2. Table 2: Amino Acids that interact with FAD Amino acid Hetero residue compound atoms Nature of interaction Val 262 Adenine nitrogens The C-O and the N-H of the Val are hydrogen bonding with the N-H and N respectively of the heterocompound. Lys 545 Ribose hydroxyl The NH of Lys 545 is hydrogen bonding to the two hydroxyls of the ribose in the adenylate 8 Jeff Hamilton Ser 101 Ile 102 Adenine nitrogen and phosphate group ribose The O-H and the N-H of Ser in hydrogen bonding to the nitrogen and the oxygen of the phosphate respectively. The –CH3 of Ile is hydrophobically interacting with the –CH2 of the ribose. Gly 103 Phosphate group The N-H of Gly is hydrogen is bonding to the oxygen of the phosphate in FAD Asn 105 Phosphate group The N-H of the amide group of Asn is hydrogen bonding to the oxygen of the phosphate of FAD. Glu 182 “ “ Asn 179 Amide group of the fused aromatic rings Arg 504 Amide group of the fused aromatic rings The amide group of Asn 179 is hydrogen bonding to the C=O of the amide group of FAD and to the hydroxyl group that is in between the nucleotide and the fused aromatic rings. The amide group of Asn 179 is hydrogen bonding to the C=O of the amide group of FAD and to the hydroxyl group that is in between the nucleotide and the fused aromatic rings. Val 185 The nitrogen in the fused aromatic rings Fused Aromatic rings “ Pro 169 Gly 184 The C=O of Val in the peptide bond is hydrogen bonding to the nitrogen in the fused aromatic rings Hydrophobic interactions between the praline methyl group and the fused aromatic rings “ Tyr 187 C=O of the fused The hydroxyl group of the tyrosine is hydrogen aromatic rings bonding to the carbonyl of the fused aromatic rings Asp 170 C=O of the fused The N-H of the amide group of Asp is hydrogen aromatic rings bonding to the carbonyl of the fused aromatic rings Trp 413 aromatic ring The aromatic Trp 413 is hydrophobically interacting with one of the fused aromatic rings. Arg 104 Phosphate group The N-H and the C=O of the peptide bond of Arg are hydrogen bonding to the oxygen of the phosphate group and the hydroxyl group that is in between the nucleotide and the fused aromatic rings. 9 Jeff Hamilton Ser 175 Phosphate group Phe 424 Fused aromatic rings The N-H of the peptide bond of Ser is hydrogen bonding to the oxygen of the phosphate group The aromatic Phe 424 is hydrophobically interacting with one of the fused aromatic rings. Gly 184 Pro 169 “ Fused aromatic rings “ The methyl of Pro is hydrophobically interacting with one of the fused aromatic rings. Glu 182 “ “ Part VII Bibliography: 1. Andrea Mattevi, Marco W. Fraaije, Alessandro Coda, Willem J.H. van Berkel Crystallization and preliminary x-ray analysis of the flavoenzyme vanillyl-alcohol oxidase from Penicillium Simplicissimum. (4):601-3. 1997 Apr. 27. http://www3.interscience.wiley.com/cgi-bin/fulltext/52413/PDFSTART 2. H.M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T.N. Bhat, H. Weissig, I.N. Shindyalov, P.E. Bourne: The Protein Data Bank. Nucleic Acids Research, 28 pp. 235-242 (2000). 3. Laskowski R A, Chistyakov V V, Thornton J M (2005). PDBsum more: new summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res., 33, D266-D268. http://www.ebi.ac.uk/pdbsum/ 10