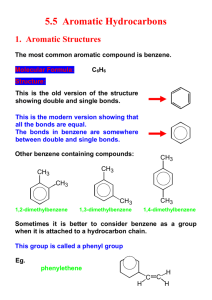
HC infrared-active molecules
advertisement

HC infrared-active molecules The following page provides extensive descriptions on fundamental principles behind the unprecedented FIR Fuel Activator® technology and interactions with your car's engine. In Organic Chemistry, hydrocarbon (HC) molecules are known to be infrared-active and can absorb multiple IR-photons causing molecular vibrations. The bond in a molecule produces an equilibrium separation between the atoms. About this equilibrium separation, a molecule can vibrate. The vibrational states are quantized. Excitation of vibrational modes in a molecule requires a photon with wavelength typically shorter than 20 μm [Ref 1]. In addition, excitation of a single mode of a molecule (so-called "fundamental") is in general much more intense than excitation of multiple modes ("combination" or "overtone" bands). Finally, a handle is also required. In this case, the critical issue is whether upon excitation of a mode, the dipole moment changes, Δρ≠0 In Organic Chemistry, hydrocarbons are known to be "IR-active". In hydrocarbons each bond may simultaneously absorb multiple IR photons at certain frequencies to induce stretching and/or bending vibrations [2]. Only vibrations that cause a change in dipole moment give rise to an absorption band on the IR spectrum (scanned between 2 μm and 16 μm), in which the larger the change in dipole moment the stronger the absorption peak. Organic chemists have been using the IR absorption spectral information, so-called Infrared Correlation Charts [3], to identify functional groups of HC’s in unknown specimens for decades. However, none of domestic industry or commercial sectors has been able to realize or test out the full potential of this innovative IR Fuel technology that leverages on the “IR-active” nature of hydrocarbons. We are proud of being the first one to discover IR-fuel excitation effect along with its applications. Based on this discovery, we invented the IR fuel saving technology that applies infrared excitation, and it works! The following page provides extensive descriptions on fundamental principles behind the unprecedented FIR Fuel Activator® technology and interactions with your car's engine. IR-absorption bands for typical hydrocarbon bonds ω (cm -1) λ (μm ) 1315 – 1475, 2800 – 3000 6.78 – 7.60, 3.33 – 3.57 1450 – 1600 6.25 – 6.90 C=C bond in aromatic ring 1620 – 1680 5.95 – 6.17 C=C 2100 – 2200 4.55 – 4.76 C≡C 3000 – 3100 3.23 – 3.33 C−H (part of aromatic ring) 3020 – 3080 3.25 – 3.31 C−H (C is ethylenic) 1420 – 1470, 1375 6.80 – 7.04, 7.27 Alkanes’ −CH3 1430 – 1470 6.80 – 6.99 Alkanes’ =C H2 1370, 1385, 1170 7.30, 7.22, 8.55 Struttura del legame C−H (in alkanes) Alkanes’ −CH(CH3) 2 910 – 920 10.87 – 10.99 *Alkenes’ RCH=CH2 880 – 900 11.11 – 11.36 *Alkenes’ R2C=CH2 675 – 730 13.70 – 14.81 *Alkenes’ cis RCH=CHR 965 – 975 10.26 – 10.36 *Alkenes’ trans RCH=CHR 730 – 770 12.99 – 13.70 *Aromatic C−H (1) 735 – 770 12.99 – 13.61 *Aromatic ortho 690 – 710 14.08 – 14.49 *Aromatic C−H (2) meta 750 – 810 12.35 – 13.33 *Aromatic C−H (2) meta 810 – 840 12.35 – 11.90 *Aromatic C−H (2) para C−H (2) NOTE: Most of the absorption bands fall in the wavelength band 3 – 14 μm that is covered by Aldi's "onesize-fits-all" FIR-emitter. Reference: 1: Turro N J, “Modern Molecular Photochemistry”, Benjamin-Cummins, Menlo Park, 1978. 2: Smith M, “Organic Chemistry”, HarperCollins Publisher, New York (1993), p. 413. 3: “Infrared Correlation Chart,” 2004 Chemical Physics Handbook, P 9-87 – 9-89.