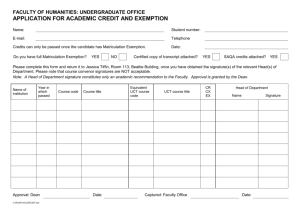
registration and abstract submission form
advertisement

Department of Human Biology UCT/MRC RESEARCH UNIT FOR EXERCISE SCIENCE & SPORTS MEDICINE Faculty of Health Sciences, University of Cape Town Private Bag, Rondebosch 7700, South Africa Tel: + 27-21-650-4561 Fax: + 27-21-686-7530 2 April 2013 Dear Staff and Postgraduate Students CALL FOR ABSTRACTS: CLINICAL LABORATORY SCIENCES AND HUMAN BIOLOGY RESEARCH DAY 2013 The Departments of Clinical Laboratory Sciences and Human Biology invite all MSc. PhD, MMed and MPhil students to present their research at our Research Day: Date: Venue: 4 September 2013 LT1 New Learning Centre, Anatomy Building. A number of prizes for the best oral and poster presentations as well as the Lafras Steyn prize for innovation will be awarded. Attached is an abstract submission form (and a sample abstract) which should be completed and submitted via email to hub-sportsinfo@uct.ac.za by no later than Monday 1 July 2013. Successful applicants will be notified via email by Friday 2 August 2013 whether their abstracts have been accepted for oral or poster presentation. All enquiries to be addressed to Debbie.Victor@uct.ac.za or telephone 021-406 6757 Yours sincerely Prof Malcolm Collins CHAIRPERSON: ORGANISING COMMITTEE Phone: 021-650 4574 e-mail: malcolm.collins@uct.ac.za _______________________________________________________________________ The University of Cape Town is committed to policies of equal opportunity and affirmative action which are essential to its mission of promoting critical inquiry and scholarship RESEARCH DAY 2013 DEPARTMENTS OF CLINICAL LABORATORY SCIENCES AND HUMAN BIOLOGY 4 SEPTEMBER 2013 LT1 LEARNING CENTRE, ANATOMY BUILDING, MEDICAL SCHOOL REGISTRATION AND ABSTRACT SUBMISSION FORM First name: Surname: Division and Department: Indicate status (tick) MBChB M.Phil M.Sc. M.Med. Ph.D. E-mail address: I am applying to present my research as (indicate preference with a tick): Oral: Poster: If your preference is not granted tick whether the alternative choice is acceptable Instructions for Abstract: (see example on next page) Body length should be NO MORE than 250 words in 11 pt Ariel font and single spaced. Full title of the presentation (IN BOLD CAPITALS) Authors and co-authors, with the name of the presenting student bold and underlined. Affiliation of authors (e.g. UCT Division or Dept. name; if co-author from another institution then name of Institution and country (but full address not required) A brief description of the work including: a) b) c) d) e) The background to the project The aims and objectives A statement concerning the methods used The main results/findings Brief discussion leading to conclusion(s) The abstract must not include references, tables or figures, nor abbreviations, except for standard abbreviations (e.g DNA, EDTA, PCR, amino acid codes etc.) or if defined in the body of the abstract. Abstracts not formatted according to the guidelines will be returned CHARACTERISATION OF THE FLAVIN ADENOSINE DINUCLEOTIDE BINDING REGION OF MYXOCOCCUS XANTHUS PROTOPORPHYRINOGEN OXIDASE CLS Studenta, AV Corrigallb, MED Technicianb, ED Sturrocka, UK Collaboratorc, PN Meissnera,b a Division of Medical Biochemistry & IIDMM Porphyria Laboratories, Dept. of Medicine c University of Cardiff, UK b Protoporphyrinogen oxidase (PPOX) catalyzes the six electron oxidation of protoporphyrinogen to protoporphyrin in haem biosynthesis. FAD is the intermediate electron acceptor and is non-covalently bound to PPOX. The FAD-binding site displays a 50-residue long signature motif typical of FAD-oxidases. In humans, mutations in PPOX may lead to variegate porphyria (VP), and the aim of this study was to rationalize potential VP-causing mutations, especially the common Arg59Trp located in the FAD-substrate interface. Based on previous studies, and structural and alignment analyses, we investigated the roles of M. xanthus PPOX residues Glu39, Trp408 and Asn441 using site-directed mutagenesis followed by expression, purification and kinetic analysis. Alteration of Glu39 to lysine and alanine resulted in an inactive enzyme while a conservative replacement (Glu39Asp) led to a more efficient enzyme. All Glu39 mutants bound FAD, except Glu39Lys. Removal of the aromatic ring in Trp408Leu led to a decrease in efficacy; and replacement of the aromatic, non-polar side chain with a polar aromatic resulted in an increased Km and 10-fold less efficient enzyme. Alteration of Asn441 to isoleucine resulted in a lower enzyme efficiency. All Trp408 and Asn441 mutants bound FAD. We conclude that the Glu39 interaction with FAD is vital, and that both Trp408 and Asn441 play roles in the FAD’s overall orientation. Specifically, the aromatic, non-polar Trp408 interaction with FAD is favoured for optimum binding of substrate and the Asn441 interaction with the ribityl group of the FAD is pivotal for the alignment of the triple ring with substrate.