Exploration 1
advertisement

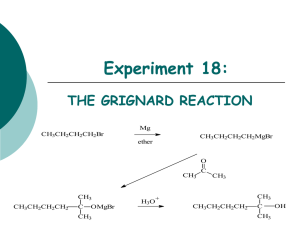
CHEM 212-2008 Experiment 4 Procedure for Weeks 1 & 2 How Can a Complex Alkene be Synthesized? R Br ? R Mg Br ? H R' O ? A. How can a Grignard Reaction be Successfully Accomplished? Pre-lab Assignment: 1. Read: This Experiment handout 2. In Your Notebook: a. Prepare a Table of Compounds including the names, mol. wts., bp’s, densities and solubilities in water and organic solvents for bromobutane, 2-methylpropanal, 2-methyl3-heptanol, diethyl ether and magnesium metal (name and mol wt. only). b. Write the Waste Disposal Instructions. Category 3: Bromobutane. Category 4: 2-Methylpropanal. Category 5: Conc. H2SO4. Category 6: All aqueous extracts. Category 7: Mg, sodium sulfate. Special: Excess ether and ether distillate -> “Recovered Ether” container in the hood. The “forerun” of the fractional distillation -> Forerun waste container. c. Using complete structural formulas of ALL organic compounds used in this experiment (R’s are not acceptable as symbols in the structures), write balanced equations for: The preparation of butylmagnesium bromide. The reaction of butylmagnesium bromide with o 2-methylpropanal (Note: -- This is an aldehyde) o water o molecular oxygen o carbon dioxide o unreacted 1-bromobutane in the dry ether solution. The reactions of aqueous sulfuric acid with: o the major organic product o the side products from the previous bullet . d. Starting on a new set of facing pages, write a complete stepwise procedure for the synthesis of 2-methyl-3-heptanol through STEP 4. # 7. e. Prepare a matching Results section including your calculated values for the volume of 1-bromobutane and 2-methylpropanal, as well as, the grams of magnesium needed for the synthesis. Include all of your calculations. 3. On separate sheets of paper complete the flow diagram for the preparation and purification of 2-methyl-3-heptanol. A partial flow diagram framework is attached at the end of this activity. Completion of the diagram requires filling in blanks and addition of boxes for some of the final purification steps. Place your flow diagram in your data binder under the Experiment 4 tab. CHEM 212 Experiment 4 2 Alkene Synthesis Introduction: For this synthesis we have selected one of the four Grignard reactions that resulted from our analysis. Thus, our synthesis will begin with 1-bromobutane and 2-methylpropanal. The preparation of the Grignard reagent, RMgX, is represented in equation (1), where RX dry ether (1) R-X + Mg R-MgX solvent represents 1-bromobutane. The exact nature of the Grignard reagent in solution is not known. It is believed to be a mixture of numerous species. These species are highly solvated by ether and are complexed with one another. It is customary to represent the Grignard reagent by the formula RMgX when writing chemical equations, but it should be kept in mind that the species in solution are of a much more complex nature. The ether solvent is an essential part of the Grignard reagent, for ether is known to form a complex with the magnesium that is present in the reagent. Several cases are known where Grignard reagents have been prepared in the absence of ether, but the yields are not good. Satisfactory yields are usually obtained when ether is present. The most common ether solvent is diethyl ether, (C2H5)2O, due to its low cost and ease of removal (its boiling point is 36 ˚C). The organic halide may, in general, be of any organic substituent (alkyl or aryl) and the halide may be bromide, chloride, or iodide. The preparation of the Grignard reagent must be carried out under anhydrous conditions and, if possible, in the absence of oxygen. It is exceedingly important to maintain completely dry conditions throughout, for the presence of water inhibits the initiation of the reaction and destroys the reagent once it is formed. The acid-base reaction that occurs when the Grignard reagent comes in contact with water is shown in equation (2). (2) R-MgX + H 2O RH + HOMgX The Grignard reagent is a strong base, since one of the carbon atoms bears substantial negative charge (R-Mg++X-). As a base, the Grignard reagent removes a proton from water. The overall effect is the hydrolysis of the reagent, with the formation of a hydrocarbon (RH) and a basic magnesium salt which coats the unreacted magnesium and inhibits further formation of the Grignard reagent. Thus it is critical to exclude water from the reaction mixture. Other weakly acidic compounds such as alcohols and carboxylic acids also destroy the Grignard reagent and inhibit the reaction by analogous acid-base reactions. In addition to the reaction with water, there are other side reactions that may occur during formation of the Grignard reagent, as shown in equations (3-5). Reaction with Oxygen: (3) RO-MgX ROO-MgX R-MgX + O 2 Reaction with Carbon Dioxide: O R-MgX + O C O R C (4) O MgX Coupling with the organic halide: (5) R-MgX + R-X R-R + MgX 2 It is possible to minimize these reactions by taking certain precautions when carrying out the experimental work. The reactions with oxygen and carbon dioxide may be avoided by carrying out the reaction under an inert atmosphere (such as nitrogen or helium gas). Also, when diethyl ether is used as a solvent, an inert gas is not essential since ether’s very high vapor pressure excludes a certain amount of air from the reaction vessel. The coupling reaction [equation (5)] is an example of a Wurtz reaction. It is not possible to eliminate this coupling reaction completely, but it may be minimized by using dilute solutions to avoid localized high concentrations of halide. This is done by using very efficient stirring and by slowly adding the halide to the magnesium in ether. Normally the rate of addition of halide Alkene Synthesis 3 CHEM 212 Experiment 4 (dissolved in ether) and the rate of reflux (when diethyl ether is used) should be adjusted so that they are about equal. Alkyl iodides are much more prone to coupling reactions than are the bromides and chlorides, so that the latter are preferable for preparing Grignard reagents even though they are less reactive. If water has been carefully excluded, the most important side reaction is the coupling process, but it is not a significant problem. Once the Grignard reagent is prepared, it is used directly in subsequent reactions. The small amount of by-products need not be separated from the reagent before adding the carbonyl compound. In our synthesis, butylmagnesium bromide is prepared from 1-bromobutane. It is then allowed to react with 2-methylpropanal to give the salt of 2-methyl-3-heptanol as shown in equation (6), and the salt is protonated with dilute sulfuric acid, equation (7). CH3 H CH3 CH2CH2CH2CH3 CH3 CH2 CH2 CH2 -MgBr + CH C CH CH (6) CH3 O CH3 O-MgBr (7) Mg+2 + HSO4- + Br- + H2O ROMgBr + H2O + H2SO4 The addition of 2-methylpropanal to the solution of butylmagnesium bromide is done slowly with stirring and, if necessary, cooling, since the reaction is highly exothermic and ether is volatile. The reaction is completed by adding dilute aqueous sulfuric acid to hydrolyze the magnesium salt and form the final organic product. In addition, the acid dissolves the insoluble basic magnesium salt that is formed. Without the acid, an unworkable emulsion may result. After the ether solution of organic products has been separated from the aqueous solution of magnesium salts, it is extracted successively with solutions of sodium bisulfite, sodium bicarbonate and sodium chloride to remove some unreacted starting material, some products of the side reactions and excess water, respectively, from the ether. (You should be able to determine the specific reactions involved in each extraction.) After further drying, the ether is removed and the product is purified by fractional distillation. Experimental Procedure Weeks 1 & 2. Synthesis. STEP 1: Calculation of reagent volumes and masses: 0.21 mole of 1-bromobutane (measure by volume) 0.20 mole of Mg. (measure by mass) 0.21 mole of 2-methylpropanal (measure by volume) STEP 2: Preparation of the Grignard Reagent: 1. Obtain the following equipment required for this step but do not assemble it until after 2. A 250 mL round bottom flask A Claisen adapter A 125 mL separatory funnel A reflux condenser Two drying tubes filled with CaCl2. A magnetic stirring bar. Two blue plastic clips BE CERTAIN THAT ALL OF THE GLASSWARE IS SCRUPULOUSLY DRY!! 2. To eliminate any possible film of moisture in the reaction flask, add the required amount of magnesium turnings and a magnetic stirring bar to the round bottom flask and attach a drying tube to the neck. Then heat the flask thoroughly by placing it in a heating well (regulator set on 5) and rotating it frequently until the entire glass surface is hot to the touch. Cool the flask to room temperature before proceeding. As the flask cools, it draws in dry air through the calcium chloride drying tube. NOTE: Cooling can be hastened by holding the flask under a stream of tap water. However, take care that no water enters the flask. 3. Set up the flask with a Claisen adapter containing a reflux condenser on the straight neck and the separatory funnel on the bent neck (see the demonstration apparatus). Leave space under the flask for your magnetic stirrer, a heating well or and ice bath, if needed. 4. Pour 10 mL of anhydrous ether slowly down the condenser into the reaction flask. CHEM 212 Experiment 4 4 Alkene Synthesis 5. Then combine the 1-bromobutane with ~30 mL of anhydrous ether, mix well and pour the solution into the separatory funnel and connect a drying tube to the top of the funnel. 6. Obtain an additional 20 mL of anhydrous ether and keep it ready to add when the reaction begins. (See 8. below) Read the following overview of the reaction initiation process. Use this overview to add cautions to the reaction steps rather than copying the overview directly into your procedure. Overview: The reaction is initiated by mixing a small portion of the alkyl halide solution with the magnesium metal. This is usually the most difficult part of the procedure. Once initiated, the reaction is strongly exothermic, so the solution must be immediately diluted with the extra 20 mL of anhydrous ether to slow the reaction down and to limit the possibilities of side reactions. When the initial burst of reaction begins to subside, additional alkyl halide solution is added slowly to maintain a controlled rate of reaction [As monitored by the boiling rate of the ether solution]. 7. Initiation of the reaction: Turn on the magnetic stirrer and admit a portion (~5 mL) of the 1-bromobutane solution into the flask. Evidence that the reaction has started will be the appearance of cloudiness and bubbling. It may take up to 5 min. for the reaction to start. If stirring is not sufficient to initiate the reaction, a second attempt, as described here, should be made. Disconnect the flask and holding it in the palm of one hand, crush a piece of magnesium against the bottom with the flattened end of a long stirring rod. Watch for bubbles indicating local boiling at that spot. You may also feel the warmth of reaction on your hand. If the reaction still fails, reassemble the apparatus, and heat the flask to boiling with your heating well and boil the solution for ~5 min. If heating also fails to initiate the reaction, ask your instructor for help. 8. As soon as the reaction starts, slowly pour the additional 20 mL of ANHYDROUS ether (See step 6. above) down the condenser to dilute the reaction mixture and thus limit the possibility of the unwanted coupling reaction. 9. When the initial reaction burst subsides, gradually add the remaining contents of the funnel by allowing the solution to drip into the reaction flask at a rate of ~1 drop per sec. (addition should take about 15 minutes) If the condenser floods badly with ether, cool the flask in an ice water bath for a few seconds only and continue. Note: When a condenser “floods”, vapor rises into it faster than the condensing liquid can flow down. The result is escape of vapor, sometimes noticeable as turbidity in the air above the condenser, and the filling of the innermost section of the condenser with roiling or turbulent liquid. 10. When the addition is complete and the reaction begins to subside, heat the mixture to continue active boiling until practically all of the Mg has dissolved (~20 min. more) or until the amount of unreacted Mg does not decrease in 5 min. STEP 3: Preparation of 2-Methyl-3-heptanol 1. While the Grignard solution is refluxing (step 10. above), measure into two different dry containers and cover to keep dry: 0.21 mole of 2-methylpropanal (measure by volume) 25 mL anhydrous ether 2. When the formation of the butyl magnesium bromide is complete, remove the heating mantle from the system and cool the flask to room temperature with an ice bath. 3. When the reaction flask is at room temperature, combine the 2-methylpropanal with the 25 mL of anhydrous ether, mix well and pour this solution into the separatory funnel that held the 1-bromobutane solution in STEP 2. 4. Turn on your magnetic stirrer and begin slow addition of the 2-methylpropanal solution to the butylmagnesium bromide solution. Be prepared to cool the flask with an ice bath if the addition leads to uncontrolled refluxing. The addition may require as much as 30 min. After the addition is complete, allow the reaction mixture to stir for an additional 10 min. 5. Place about 150 mL of crushed ice into a 500 mL beaker and add 9 mL of concentrated sulfuric acid. In a hood, pour the reaction mixture slowly and with stirring into the ice-acid Alkene Synthesis 5 CHEM 212 Experiment 4 mixture. Continue stirring the mixture until all remaining magnesium has reacted and dissolved. After the addition is complete, transfer the cold mixture, which may contain some precipitate, to a separatory funnel and shake it gently. The precipitate should dissolve. 6. Separate the layers transferring the ether layer to an Erlenmeyer flask. 7. Extract the aqueous layer with two 25-mL portions of ether and add these ether extracts to the original ether layer. 8. Rinse the Claisen head and reaction flask with 6 M HCl. Allow the flask to sit with the HCl until all remaining magnesium has reacted and dissolved. Then rinse the Claisen head and reaction flask with water and acetone. Do NOT rinse the condenser or separatory funnel. Return all glassware to the appropriate bins. 9. The procedure may be stopped at this point if necessary. If so, transfer the combined ether layers to an Erlenmeyer flask and stopper it tightly with a cork. STEP 4: Preliminary Purification 1. Extract the combined ether solutions (in the separatory funnel) with 30 mL of saturated sodium bisulfite solution venting the funnel to relieve pressure. 2. Extract the ether layer with two 30-mL portions of 10% sodium bicarbonate solution, again venting the funnel frequently. 3. Test the pH of the aqueous phase after the second sodium bicarbonate extraction. The solution should be as basic as the original sodium bicarbonate solution used for the extraction. 4. If the second extract is not basic enough, extract the ether solution with another 30 mL portion of sodium bicarbonate and continue these extractions until the aqueous phase tests appropriately basic after the extraction. 5. Extract the ether solution with 30 mL of saturated sodium chloride solution. 6. Remove the remainder of the water by drying the ether solution over anhydrous sodium sulfate. 7. If necessary, stopper the flask snugly with a cork, mark the solvent level and allow the solution to stand over the drying agent until the next lab period. STEP 5: Final Purification. 1. Filter the solution from the drying agent through a fluted filter into a 250 mL round bottom flask (If the volume of the solution has decreased significantly, you may need to add some ether so that the filtration can be accomplished). 2. Remove most of the ether from the product by simple distillation (remember the boiling chip). 3. When the vapor temperature rises about 20 ˚C above the bp of ether, stop the simple distillation. The volume of solution left in the pot should be about 25 mL. The distillate, recovered ether, should be placed in the bottles marked for it. 4. Transfer the contents of the simple distillation pot to a 50 mL round bottom flask, rinse the original flask with 3 mL of your simple distillation distillate (“Recovered Ether”), and convert your simple distillation apparatus to a fractional one by adding a column packed with glass beads. 5. Be sure that the thermometer bulb is properly adjusted in the still head. Insulate the flask and still head with glass wool and Al foil, and continue distilling. 6. Collect any forerun boiling more than 3 ˚C below the product’s bp. separately from the product, record the boiling range and approximate volume of the forerun then collect a product fraction with a boiling range of ~5 ˚C or less. 7. Record the boiling range and approximate volume of the product. If your yield of 2-methyl-3-heptanol is less than 8 mL, check with your instructor. Your thermometer may be incorrectly placed. 8. Weigh the product and calculate the % yield. 9. Place the product in a labeled vial or flask and keep it tightly stoppered for the Week 3 & 4 procedure. Discard the forerun in forerun waste container. CHEM 212 Experiment 4 MH-OH Bu 6 Alkene Synthesis Partial Flow Diagram Framework for the Synthesis and Purification of 2-Methyl-3-Heptanol Abbreviations = 2-methyl-3-heptanol E = ether = C4H9— 2-mp = 2-methylpropanal = missing entry This diagram assumes that a trace of water is present in the original reaction. E, Bu-Br, Mg, O2(trace), H2O(trace) reflux Gas Phase Bu-H First Reaction Solution E, Mg, Bu-Br, BuOMgBr, BuBu, BuCO2MgBr, O2(trace)) Add 2-mp Second Reaction Solution Gas Phase H2 , Bu-H E, Mg, Bu-Br, 2-mp, O2(trace) 1. H2O, H2SO4 2. Ether extraction 3. Combine extracts Aqueous H2O, E(trace) H3O+, HSO4Mg2+ E, Bu-Br, 2-mp, H2O, O2(trace) Ether NaHSO3 extraction Aqueous H2O, E(trace) + Na , HSO3- Ether E, H2O, O2(trace) Complete the flow diagram by adding other "boxes" for extraction and distillation steps