AP Chemistry Unit 8- Homework Problems Ka and Kb Acid & Base
advertisement
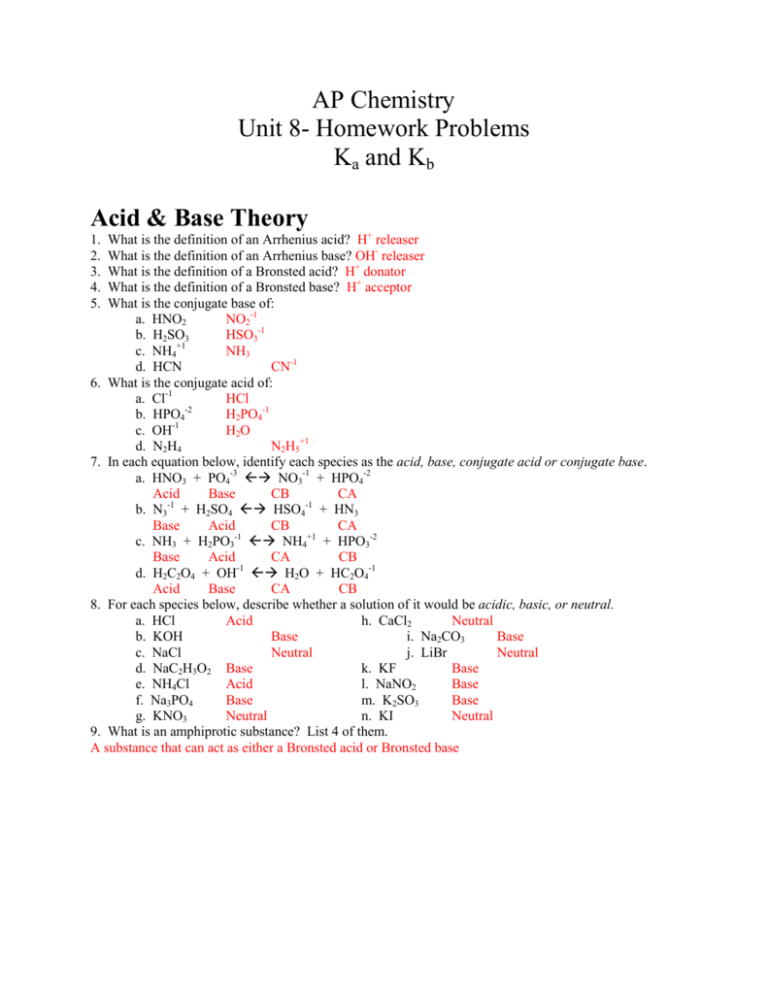
AP Chemistry Unit 8- Homework Problems Ka and Kb Acid & Base Theory What is the definition of an Arrhenius acid? H+ releaser What is the definition of an Arrhenius base? OH- releaser What is the definition of a Bronsted acid? H+ donator What is the definition of a Bronsted base? H+ acceptor What is the conjugate base of: a. HNO2 NO2-1 b. H2SO3 HSO3-1 +1 c. NH4 NH3 d. HCN CN-1 6. What is the conjugate acid of: a. Cl-1 HCl b. HPO4-2 H2PO4-1 -1 c. OH H2O d. N2H4 N2H5+1 7. In each equation below, identify each species as the acid, base, conjugate acid or conjugate base. a. HNO3 + PO4-3 NO3-1 + HPO4-2 Acid Base CB CA b. N3-1 + H2SO4 HSO4-1 + HN3 Base Acid CB CA c. NH3 + H2PO3-1 NH4+1 + HPO3-2 Base Acid CA CB -1 d. H2C2O4 + OH H2O + HC2O4-1 Acid Base CA CB 8. For each species below, describe whether a solution of it would be acidic, basic, or neutral. a. HCl Acid h. CaCl2 Neutral b. KOH Base i. Na2CO3 Base c. NaCl Neutral j. LiBr Neutral d. NaC2H3O2 Base k. KF Base e. NH4Cl Acid l. NaNO2 Base f. Na3PO4 Base m. K2SO3 Base g. KNO3 Neutral n. KI Neutral 9. What is an amphiprotic substance? List 4 of them. A substance that can act as either a Bronsted acid or Bronsted base 1. 2. 3. 4. 5. Auto-Ionization of water, pH, and strong acids 1. 2. 3. 4. What is the equation for the auto-ionization of water? 2 H2O (l) H3O+1 (aq) + OH-1 (aq) What is the equation for Kw and what is its value? Kw = [H3O+][OH-1] = 1x10-14 Because the pH scale is a logarithmic scale, every change in 1 is really a change in how much? 10 Using the pH square and the equations learned in class, fill in the blank spots in the chart below: pH [H3O+1] [OH-1] pOH -4 -11 3.5 3.16x10 3.16x10 10.5 8.04 9.2x10-9 1.10x10-6 5.96 3.67 2.14x10-4 4.7x10-11 10.33 5.8 1.58x10-6 6.31x10-9 8.2 5. According to the leveling effect: a. What is the strongest acid species in water? H3O+1 b. What is the strongest base species in water? OH-1 6. What is the % dissociation of strong acids and bases in water? 100% 7. List 6 strong acids. HCl, HBr, HI, HNO3, HClO4, H2SO4 8. List 6 strong bases. LiOH, NaOH, KOH, RbOH, CsOH, Ba(OH)2, Sr(OH)2 Weak acids & bases, Ka, Kb, and pH 1. 2. 3. 4. I C E 5. I C E How are weak acids and bases different from strong ones? Weak acids dissociate <5% What is the general equation for Ka? HA (aq) + H2O (l) H3O+1 (aq) + A-1 (aq) What is the general equation for Kb? B (aq) + H2O (l) OH-1 (aq) + HB+1 (aq) For a 0.25 M solution of HC2H3O2 (Ka = 1.8x10-5) calculate: a. pH HA (aq) + H2O (l) H3O+1 (aq) + A-1 (aq) 0.25 0 0 -x +x +x 0.25 x x 1.8x10-5 = x2/0.25 x = 2.12x10-3 pH = -log (2.12x10-3) = 2.67 b. % dissociation 2.12x10-3 x 100% = 0.85% 0.25 For a 0.75 M solution of C2H5NH2 (Kb = 5.6x10-4) calculate: a. pH B (aq) + H2O (l) OH-1 (aq) + HB+1 (aq) 0.75 0 0 -x +x +x 0.75 x x 5.6x10-4 = x2/0.75 x = 2.05x10-2 pOH = -log (2.05x10-2) = 1.69 pH = 12.31 b. % dissociation 2.05x10-2 x 100% = 2.73% 0.75 6. For a 0.055 M solution of HClO (Ka = 2.9x10-8) calculate: a. pH HA (aq) + H2O (l) H3O+1 (aq) + A-1 (aq) I 0.055 0 0 C -x +x +x E 0.055 x x 2.9x10-8 = x2/0.055 x = 3.99x10-5 pH = -log (3.99x10-5) = 4.40 b. % dissociation 3.99x10-5 x 100% = 0.0725% 0.055 7. For a 0.0034 M solution of C5H5N (Kb = 1.7x10-9) calculate: a. pH B (aq) + H2O (l) OH-1 (aq) + HB+1 (aq) I 0.0034 0 0 C -x +x +x E 0.0034 x x 1.7x10-9 = x2/0.0034 x = 2.40x10-6 pOH = -log (2.40x10-6) = 5.62 pH = 8.38 b. % dissociation 2.40x10-6 x 100% = 0.071% 0.0034 8. For a 0.15 M solution of H2C6H6O6 (Ka1 = 8x10-5 Ka2 = 1.6 x10-12) calculate: a. pH H2A (aq) + H2O (l) H3O+1 (aq) + HA-1 (aq) I 0.15 0 0 C -x +x +x E 0.15 x x 8x10-5 = x2/0.15 x = 3.46x10-3 pH = -log (3.46x10-3) = 2.46 b. Equilibrium concentrations of: i) HC6H6O6-1 x = 3.46x10-3 ii) C6H6O6-2 Ka2 = 1.6x10-12 9. For a 0.025 M solution of diprotic base, B (Kb1 = 4x10-6, Kb2 = 8x10-10) calculate: a. pH B (aq) + H2O (l) OH-1 (aq) + HB+1 (aq) I 0.025 0 0 C -x +x +x E 0.025 x x 4x10-6 = x2/0.025 x = 3.16x10-4 pOH = -log (3.16x10-4) = 3.5 pH = 10.5 b. Equilibrium concentrations of: i) HB+ x = 3.16x10-4 ii) H2B+2 Kb2 = 8x10-10 Acid-Base Interactions Strong-Strong 1. What is the pH when 50 mL of 0.1 M HCl is mixed with 50 mL of 0.1 M NaOH? Acid = (50/100)(0.1 M) = 0.05 M Base = (50/100)(0.1 M) = 0.05 M Equal amounts of strong acid/base so should be neutral H3O+1 (aq) + OH-1 (l) 2 H2O I 0.05 0.05 C -0.05 -0.05 E 0 0 pH = 7 2. What is the pH when 250 mL of 0.2 M HNO3 is mixed with 500 mL of 0.1 M KOH? Acid = (250/750)(0.2 M) = 0.0667 M Base = (500/750)(0.1 M) = 0.0667 M Equal amounts of strong acid/base so should be neutral I C E H3O+1 (aq) + 0.0667 -0.0667 0 pH = 7 OH-1 (l) 2 H2O 0.0667 -0.0667 0 3. What is the pH when 100 mL of 0.001 M HBr is mixed with 50 mL of 0.001 M NaOH? Acid = (100/150)(0.001 M) = 6.67x10-4 M Base = (50/150)(0.001 M) = 3.33x10-4 M More acid than base so should be acidic I C E H3O+1 (aq) + OH-1 (l) 2 H2O 6.67x10-4 3.33x10-4 -4 -3.33x10 -3.33x10-4 -4 3.33x10 0 -4 pH = -log (3.33x10 ) = 3.47 4. What is the pH when 40 mL of 0.01 M HI is mixed with 120 mL of 0.008 M CsOH? Acid = (40/160)(0.01 M) = 0.0025 M Base = (120/160)(0.008 M) = 0.006 M More base than acid so should be basic I C E H3O+1 (aq) + OH-1 (l) 2 H2O 0.0025 0.006 -0.0025 -0.0025 0 0.0035 pOH = -log (0.0035) = 2.46 pH = 11.54 5. What is the pH when 50 mL of 0.0025 M H2SO4 is mixed with 75 mL of 0.0040 M LiOH? Acid = 2*(50/125)(0.0025 M) = 0.0020 M Base = (75/125)(0.0040 M) = 0.0024 M More base than acid so should be basic I C E H3O+1 (aq) + OH-1 (l) 2 H2O 0.0020 0.0024 -0.0020 -0.0020 0 0.0004 pOH = -log (0.0004) = 3.4 pH = 10.6 6. What is the pH when 20 mL of 0.00050 M HClO4 is mixed with 20 mL of 0.00040 M Ba(OH)2? Acid = (20/40)(0.00050 M) = 0.00025 M Base = 2* (20/40)(0.0004 M) = 0.0004 M More base than acid so should be basic I C E H3O+1 (aq) + OH-1 (l) 2 H2O 0.00025 0.0004 -0.00025 -0.00025 0 0.00015 pOH = -log (0.00015) = 3.8 pH = 10.2 Strong-Weak 7. What is the pH when 25 mL of 0.25 M HCl is mixed with 25 mL of 0.25 M NH3? (Kb = 1.8x10-5)? Acid = (25/50)(0.25 M) = 0.125 M Base = (25/50)(0.25 M) = 0.125 M Equal amounts of strong acid/weak base so should be acidic Shortcut hint- Equivalence point of a strong acid weak base gives a normal Ka problem (you can skip the actual rxn and go straight to Ka of conj. acid of weak base) ACTUAL RXN- GOES ALMOST ALL THE WAY (water is a product; math is bad) H3O+1 (aq) + NH3 (aq) H2O (l) + NH4+1 (aq) I 0.125 0.125 0 C -0.125 -0.125 +0.125 E 0 0 0.125 NOW FLIP RXN AND DO Ka (Ka = 1x10-14/1.8x10-5) = 5.56x10-10 NH4+1 (aq)+ H2O (l) H3O+1 (aq) + NH3 (aq) I 0.125 0 0 C -x +x +x E 0.125 x x 5.56x10-10 = x2/0.125 x = 8.33x10-6 pH = -log (8.33x10-6) = 5.08 8. What is the pH when 20 mL of 0.020 M HNO3 is mixed with 20 mL of 0.030 M C6H5NH2 (Kb = 4x10-10)? Acid = (20/40)(0.02 M) = 0.01 M Base = (20/40)(0.030 M) = 0.015 M Not at equivalence point. Not at midpoint. ACTUAL RXN- GOES ALMOST ALL THE WAY (water is a product; math is bad) +1 H3O+1 (aq) + C6H5NH2 (aq) H2O (l) + C6H5NH3 (aq) I 0.01 0.015 0 C -0.01 -0.01 +0.01 E 0 0.005 0.01 NOW FLIP RXN AND DO Ka (Ka = 1x10-14/4x10-10) = 2.5x10-5 +1 C6H5NH3 (aq) + H2O (l) H3O+1 (aq) + C6H5NH2 (aq) I 0.01 0 0.005 C -x +x +x E 0.01 x 0.005 2.5x10-5 = x(0.005/0.01) x = 5x10-5 pH = -log (5x10-5) = 4.30 Shortcut hint- Simple buffer problem. Write Ka equation and plug in amounts after strong acid is neutralized (you can skip the actual rxn) Strong acid: 0.01 M neutralized is turned into a weak acid Weak base: 0.015 M -0.01 M neutralized by acid so 0.005 left Now plug straight into 2nd ICE table above and solve 9. What is the pH when 50 mL of 0.040 M NaOH is mixed with 50 mL of 0.040 M HN3 (Ka = 1.9x10-5)? Base = (50/100)(0.04 M) = 0.02 M Acid = (50/100)(0.04 M) = 0.02 M Equal amounts of strong base/weak acid so should be basic ACTUAL RXN- GOES ALMOST ALL THE WAY (water is a product; math is bad) -1 OH-1 (aq) + HN3 (aq) H2O (l) + N3 (aq) I 0.02 0.02 0 C -0.02 -0.02 +0.02 E 0 0 0.02 NOW FLIP RXN AND DO Kb (Kb = 1x10-14/1.9x10-5) = 5.26x10-10 -1 N3 (aq)+ H2O (l) OH-1 (aq) + HN3 (aq) I 0.02 0 0 C -x +x +x E 0.02 x x 5.26x10-10 = x2/0.02 x = 3.24x10-6 pOH = -log (3.242x10-6) = 5.49 pH = 8.51 Shortcut hint- Equivalence point of a strong base weak acid gives a normal Kb problem (you can skip the actual rxn and go straight to Kb of conj. base of weak acid) 10. What is the pH when 120 mL of 0.0075 M KOH is mixed with 200 mL of 0.0050 M HOCl (Ka = 3.5x10-8)? Base = (120/320)(0.0075 M) = 0.00281 M Acid = (200/320)(0.005 M) = 0.00312 M Not at equivalence point. Not at midpoint. ACTUAL RXN- GOES ALMOST ALL THE WAY (water is a product; math is bad) -1 OH-1 (aq) + HOCl (aq) H2O (l) + OCl (aq) I 0.00281 0.00312 0 C -0.00281 -0.00281 +0.00281 E 0 0.00031 0.00281 NOW FLIP RXN AND DO Kb (Kb = 1x10-14/3.5x10-8) = 2.89x10-7 -1 OCl (aq) + H2O (l) OH-1 (aq) + HOCl (aq) I 0.00281 0 0.00031 C -x +x +x E 0.00281 x 0.00031 2.89x10-7= x(0.00031/0.00281) pOH = -log (2.59x10-6) = 5.59 x = 2.59x10-6 pH = 8.41 Shortcut hint- Simple buffer problem. Write Ka equation and plug in amounts after strong base is neutralized (you can skip the actual rxn) Strong base = 0.00281 turns into weak base Weak acid = 0.00312 neutralized and left with 0.00312-0.00281 = 0.00031 So: HOCl (aq) + H2O (l) H3O+ (aq) + OCl-1 (aq) I 0.00031 0 0.00281 C -x +x +x E 0.00031 x 0.00281 3.5x10-8 = x(0.00281/0.00031) x = 3.86x10-9 pH = 8.41 Midpoint 11. What is the pH when 25 mL of 0.050 M HCl is mixed with 50 mL of 0.050 C5H5N (Kb = 1.5x10-9)? acid = (25/75)(0.050 M) = 0.0166 M base = (50/75)(0.050 M) = 0.0333 M At midpoint! (Half as much strong acid as base) pH = pKa so Ka = (1x10-14/1.5x10-9) = 6.67x10-6 so pH = - log (6.67x10-6) = 5.18 12. What is the pH when 40 mL of 0.80 M NaOH is mixed with 80 mL of 0.8 M HNO2 (Ka = 4.5x10-4)? base = (40/120)(0.80 M) = 0.266 M acid = (80/120)(0.80 M) = 0.5333 M At midpoint! (Half as much strong base as acid) pH = pKa so pH = - log (4.5x10-4) = 3.35 13. What is the pH when 30 mL of 0.006 M HNO3 is mixed with 30 mL of 0.012 M (CH3)3N (Kb = 7.4x10-5)? acid = (30/60)(0.006 M) = 0.003 M base = (30/60)(0.012 M) = 0.006 M At midpoint! (Half as much strong acid as base) pH = pKa so Ka = (1x10-14/7.4x10-5) = 1.35x10-10 so pH = - log (1.35x10-10) = 9.87 14. What is the pH when 100 mL of 0.0078 M KOH is mixed with 100 mL of 0.0156 M C6H5CO2H (Ka = 6.3x10-5) base = (100/200)(0.0078 M) = 0.0039 M acid = (100/200)(0.0156 M) = 0.0078 M At midpoint! (Half as much strong base as acid) pH = pKa so pH = - log (6.3x10-5) = 4.2 Ksp and pH 1. What is the pH of a saturated solution of Mn(OH)2 Ksp = 1.9x10-13? Ksp = [Mn+2][OH-1]2 1.9x10-13 = 4x3 x= 3.67x10-5 so [OH-1] = 7.32 x10-5 so pOH = 4.14 so pH = 9.86 2. What is the pH of a saturated solution of Ca(OH)2 Ksp = 5.5x10-5? Ksp = [Ca+2][OH-1]2 5.5x10-5 = 4x3 x= 0.0240 so [OH-1] = 0.0480 so pOH = 1.32 so pH = 12.68 3. At what pH will a 0.0075 M solution of Mg(OH)2 begin to form a precipitate (Ksp = 5.6x10-12)? Ksp = [Mg+2][OH-1]2 5.6x10-12 = [0.0075][x] 2 x= 2.73x10-5 so [OH-1] = 2.73x10-5 so pOH = 4.56 so pH = 9.44 4. At what pH will a 0.00042 M solution of Pb(OH)2 begin to form a precipitate (Ksp = 1.4x10-15)? Ksp = [Pb+2][OH-1]2 1.4x10-15 = [0.00042][x] 2 x= 1.83x10-6 so [OH-1] = 1.83x10-6 so pOH = 5.74 so pH = 8.26 Titration Curves 1. Draw a titration curve of a strong base being titrated by a strong acid. Be sure to label a pH of 7 in the appropriate spot. pH 7 mL acid added 2. Draw a titration curve of a strong acid being titrated by a strong base. Be sure to label a pH of 7 in the appropriate spot. pH 7 mL acid added 3. Draw a titration curve of a weak base being titrated by a strong acid. Be sure to label a pH of 7 in the appropriate spot. pH 7 mL acid added 4. Draw a titration curve of a weak acid being titrated by a strong base. Be sure to label a pH of 7 in the appropriate spot. pH 7 mL acid added 7. What is the significance of the midpoint of a titration? It is pH = pKa 8. If 40 mL of weak acid HA was titrated as the graph below shows, calculate: a. Show the pH at the equivalence point Eq. Pt @30 mL pH = 8.5 b. Show the pH at the midpoint Mid Pt @15 mL pH = 5 c. Calculate the original M of HA MaVa = MbVb Ma(40 mL) = (0.10 M)(30 mL) Ma = 0.075 M d. Calculate Ka of HA pKa = pH at midpoint so pKa = 5 or Ka = 1x10-5 Titration of 40 mL of ??? M weak acid HA with 0.10 M NaOH Eq. Pt @30 mL pH = 8.5 Mid Pt @15 mL pH = 5 9. If 25 mL of weak base B was titrated as the graph below shows, calculate: a. Show the pH at the equivalence point Eq. Pt @60 mL pH = 4 b. Show the pH at the midpoint Mid Pt @ 30 mL pH = 8 c. Calculate the original M of B MaVa = MbVb (0.50 M)(60 mL) = (? M)(25 mL) Mb = 1.2 M d. Calculate Kb of B pKa = pH at midpoint so pKb = pOH at midpoint or Kb = 1x10-6 Titration of 25 mL of ??? M weak base B with 0.50 M HCl Mid Pt @ 30 mL pH = 8 Eq. Pt @60 mL pH = 4 HCl Buffers 1. What is the definition of a buffer? A solution that resists changes in pH. 2. What kind of an acid and base are present in a buffer? Weak acid and conj base or weak base and conj acid 3. The Ka of carbonic acid is 4.3x10-7. a. What is the pH of a solution that is 0.25 M H2CO3 and 0.25 M NaHCO3? -log (4.3x10-7) = 6.4 b. What would the ratio of acid to base be if you wanted to buffer at exactly a pH of 6? 4.3x10-7 = 1x10-6 (B/A) B:A = 0.43:1 or A:B = 2.32:1 4. The Ka of hydrozoic acid is 1.9x10-5. a. What is the pH of a solution that is 0.25 M HN3 and 0.75 M N3-1? Ka = 1.9x10-5 = [H3O+1](0.25/0.75) = 5.7x10-5 pH = -log(5.7x10-5) = 4.24 b. What would the ratio of acid to base be if you want to buffer exactly at a pH of 5? 1.9x10-5 = 1x10-5 (B/A) B:A = 1.9:1 or A:B = 0.53:1 -10 5. The Kb of aniline is 4x10 . a. What is the pH of a solution that is 0.050 M C6H5NH2 and 0.050 M C6H5NH3+1? pOH = -log (4x10-10) = 9.4 so pH = 4.6 or pH = -log (2.5x10-5) = 4.6 b. What would the ratio of acid to base be if you want to buffer exactly at a pH of 5? 2.5x10-5 = 1x10-5 (B/A) B:A = 2.5:1 or A:B = 0.4:1 -4 6. The Kb of ethyleamine is 4.4x10 . a. What is the pH of a solution that is 0.25 M C5H5N and 1.25 M C5H5NH+1? Ka = 1x10-14/4.4x10-4 = 2.27x10-11 2.27x10-11 = [H3O+1] (0.25/1.25) = 1.14x10-10 pH = -log(1.14x10-10) = 9.95 b. What would the ratio of acid to base be if you want to buffer exactly at a pH of 10? 2.5x10-11 = 1x10-10 (B/A) B:A = 0.25:1 or A:B = 4:1 7. Which of the following buffer systems would you choose if you want to buffer at a pH of 2? a. HC3H5O2 / C3H5O2-1 Ka = 1.35x10-5 -1 b. HNO2 / NO2 Ka = 7.2x10-4 c. HIO / IO-1 Ka = 2.3x10-11 -1 d. H3PO4 / H2PO4 Ka = 7.6x10-3 You would pick system d. The Ka value is closest to the pH you want to buffer at. Describe how to make the buffer so it is exactly at a pH of 2. 7.6x10-3 = 1x10-2 (B/A) so: B:A = 0.76:1 or A:B = 1.316 8. Lactic acid has a Ka of 8.3x10-4. A buffer is made so that it is 0.50 M in HC3H5O3 and 0.50 M in C3H5O3-1. a. What is the pH of the buffer? pH = -log(8.3x10-4) = 3.1 b. What will the pH of the buffer be if 0.15 M of HCl are added? 0.15 M HCl will react away 0.15 M of the weak base and add 0.15 M of the weak acid 8.3x10-4 = [H3O+1](0.35/0.65) = 1.54x10-3 pH = -log(1.54x10-3) = 2.8 9. Arsenous acid has a Ka of 6.6x10-10. A solution is made so that it is 50 mL of 1.2 M in HAsO2. a. What is the pH of the solution? 6.6x10-10 = x2/1.2 x = [H3O+1] = 2.8x10-5 pH = 4.55 b. How many grams of NaAsO2 must be added so the solution is a buffer at a pH of 10? Assume no change in volume. 6.6x10-10 = 1x10-10 ([base]/1.2 M acid) so [base] = 7.92 M [base] = 7.92 M so 7.92 moles/L * 0.05 L = 0.396 moles NaAsO2 0.396 moles NaAsO2 * 129.9 g/mole = 51.4 g NaAsO2 10. Methylamine has a Kb of 4.2x10-4. A buffer is made so that it is 0.75 M in CH3NH2 and 0.75 M in CH3NH3+1. a. What is the pH of the buffer? pOH = -log(4.2x10-4) = 3.4 so pH = 10.6 or pH = -log(2.4x10-11) = 10.6 b. What will the pH of the buffer be if 0.25 M of HNO3 are added? 0. 25 M HNO3 will react away 0.25 M of the weak base and add 0.25 M of the weak acid 2.4x10-11 = [H3O+1](0.50/1.0) = 4.8x10-11 pH = -log(4.8x10-11) = 10.3 11. Quinoline has a Kb of 6.3x10-10. A solution is made so that it is 250 mL of 1.75 M C9H7N. a. What is the pH of the solution? 6.3x10-10 = x2/1.75 x = 3.32x10-5 pOH = 4.48 pH = 9.52 b. How many grams of C9H7NHCl must be added so that the solution is a buffer exactly at a pH of 5? Assume no change in volume. Ka = 1x10-14/6.3x10-10 = 1.59x10-5 1.59x10-5 = 1x10-5(1.75 M/[acid]) so [acid] = 1.1 M [acid] = 1.1 M so 1.1 moles/L * 0.25 L = 0.275 moles acid 0.275 moles acid * 165.5 g/mole = 45.5 grams C9H7NHCl Lewis Acids & Bases 1. 2. 3. 4. What is the definition of a Lewis Acid? Electron pair acceptor What is the definition of a Lewis Base? Electron pair donor What is an amphoteric substance? A substance that can be a Bronsted base or a Lewis acid Give 4 examples of amphoteric substances. Al(OH)3 Zn(OH)2 Cr(OH)3 Sn(OH)4 5. Aluminum hydroxide, Al(OH)3, is a famous amphoteric substance. a. Write an equation showing Al(OH)3 acting as a Bronsted base Al(OH)3 + 3 H3O+1 6 H2O + Al+3 b. Write an equation showing Al(OH)3 acting as a Lewis acid Al(OH)3 + OH-1 [Al(OH)4]-1 6. In the following equations, which species is acting as the Lewis acid and the Lewis base? a. NH3 + BF3 BF3NH3 LB LA b. SnCl4 + 2 Cl-1 [SnCl6]-2 LA LB c. H+ + LA OH-1 H2O LB Combination Problems 1. a) A 0.10 M solution of it has a starting pH of 3. If it was a strong acid it should have a pH of 1. Also, the equivalence point is at about a pH of 9. It it was a strong acid, the equivalence point should be at 7 through this titration with a strong base NaOH. b) Find the pH at the midpoint of the titration. The equivalence point is at 25 so the midpoint is at 12.5 mL. The pH at this point is 5 so pKa = 5 so Ka = 1x10-5 c) See above line in red d) i) MaVa = MbVb (0.10)(25 mL) = (0.20 M)(x mL Vb = 12.5 mL ii) Less NaOH will be needed so the concentration will be higher resulting in a more concentrated base and so a higher equivalence point 2. a) NH3 + H3O+1 NH4+1 + H2O b) Starting pH: 1.8x10-5 = x2/0.10 x = 0.00134 pOH = 2.87 pH = 11.13 Equivalence point: (30 mL) (0.1 M) = (x mL)(0.2 M) x mL = 15 mL NH3 at equivalence point: (0.10 M)(30/45) = 0.0667 M HCl at equivalence point: (0.20 M)(15/45) = 0.0667 M pH at equivalence point: 1x10-14/1.8x10-5 = x2/0.0667 x = 6.1x10-6 pH = 5.2 c) Since the equivalence point is at 4.71, methyl red will be the closest to that as it changes color at a pH of 5.5 d) Since Kb for NH3 is 1.8x10-5 that means that Ka for NH4+1 is 1x10-14/1.8x10-5 = 5.6x10-10. Since Kb is larger than Ka, the solution will be basic 3. For the reaction: NH3 (aq) + H2O (l) NH4+1 (aq) + OH-1 (aq) In 0.0180 M NH3 at 25 oC, the [OH-1] is 5.6x10-4M. a) Write the equilibrium-constant expression for the reaction above. Kb = [NH4+1][OH-1]/[NH3] b) Determine the pH of 0.0180 M NH3 pOH = -log (5.6x10-4) = 3.25 pH = 10.75 c) Determine the value of Kb for NH3 Kb = (5.6x10-4)2/(0.0180) = 1.74x10-5 d) Determine the % ionization of NH3 % Ionization = (5.6x10-4)/(0.0180) *100% = 3.11% e) In an experiment, a 20.0 mL sample of 0.0180 M NH3 was placed in a flask and titrated to the equivalence point and beyond using 0.0120 M HCl i) Determine the volume of 0.0120 M HCl that was added to reach the equivalence pt MaVa = MbVb (0.0120)(Va) = (0.0180)(20 mL) Va = 30 mL ii) Determine the pH of the solution in the flask after a total of 15.0 mL of 0.0120 M HCl was added This is the midpoint of the titration so the midpoint pH = pKa Kb = 1.74x10-5 so Ka = 1x10-14/1.74x10-5 = 5.747x10-10 pH = -log (5.747x10-10) = 9.24 iii) Determine the pH of the solution in the flask after a total of 40.0 mL of 0.0120 M was added. HCl: 0.0120 M (40/60) = 0.008 M NH3: 0.0180 M (20/60) = 0.006 M H3O+1 + NH3 NH4+1 + H2O 0.008 0.006 0.002 0 pH = log (0.002) = 2.70 4. For the reaction: C6H5NH2 (aq) + H2O (l) C6H5NH3+1 (aq) + OH-1 (aq) a) Write the equilibrium constant expression, Kb, for the reaction above. Kb = [C6H5NH3+1][OH-1]/[C6H5NH2] b) A sample of C6H5NH2 is dissolved in water to produce 25.0 mL of a 0.010 M solution. The pH of the solution is 8.82. Calculate Kb. b) pH = 8.82 []o []eq [OH-1] = 10-5.18 = 6.61 x10-6 pOH = 5.18 C6H5NH2 + 0.10 -6.61 x10-6 0.1 H2O C6H5NH3+ + 0 +6.61 x10-6 6.61 x10-6 OH0 +6.61 x10-6 6.61 x10-6 Kb = (6.61 x10-6)2/0.1 = 4.37 x10-10 c) The solution prepared in part (b) is titrated with 0.10 M HCl. Calclulate the pH of the solution when 5.0 mL of the acid has been added. c) Base = (0.1) (25/30) = 0.0833 Acid = (0.1) (5/30) = 0.0167 H3O+ 0.0167 -0.0167 0 C6H5NH3+ + 0 +0.0167 0.0167 H2O []o []eq C6H5NH2 + 0.0833 -0.0167 0.0666 H2O []o []eq C6H5NH2 + 0.0666 -x 0.0666 C6H5NH3+ + 0.0167 +x 0.0167 OH0 +x x 4.37 x10-10 = [C6H5NH3+][OH-1]/[ C6H5NH2] 4.37 x10-10 = [0.0167][x]/[ 0.0666] X = 1.74 x10-9 pOH = -log 1.74 x10-9 = 8.76 pH = 5.24 d) Calculate the pH at the equivalence point of the titration in (c) H3O+ 0.05 -0.05 0 C6H5NH3+ + 0 +0.05 0.05 H2O []o []eq C6H5NH2 + 0.05 -0.05 0 H2O []o []eq C6H5NH3+ + 0.05 -x 0.05 C6H5NH2 + 0 +x x H3O+ 0 +x x d) Ka = 1x10-14/4.37x10-10 = 2.29 x10-5 X = 0.00107 pH = -log 0.00107 = 2.97 x2 /0.05 = 2.29 x10-5 = Ka e) The pKa values for several indicators are given below. Which is most suitable for this titration? Justify. Erythrosine pKa = 3 Litmus pKa = 7 Thymolphthalein pKa = 10 e) As the pH changes at about 3, erythrosine is the best as it changes at the same pH value. 5. Hypochlorous acid, HOCl, is a weak acid. The Ka for HOCl is: Ka = [H3O+1][OCl-1] = 3.2x10-8 [HOCl] a) Write a chemical equation showing how HOCl behaves as an acid in water. a) HOCl (aq) + H2O (l) H3O+ (aq) + OCl-1 (aq) b) Calculate the pH of a 0.175 M solution of HOCl 3.2 x10-8 = x2/0.175 x = 7.48 x10-5 pH = -log x = log 7.48 x10-5 = 4.13 c) Write the net ionic equation for the reaction between HOCl and NaOH c) HOCl (aq) + OH-1 (aq) H2O (l) + OCl-1 (aq) d) In an experiment, 20.00 mL of 0.175 M HOCl is titrated with 6.55 mL of 0.435 M NaOH i) Calculate the number of moles of NaOH added di) 0.0655 L x 0.435 moles/L = 0.0285 moles OH- added ii) Calculate the [H3O+1] in the flask after the NaOH has been added dii) []o []eq acid = 0.175 (20/26.55) = 0.132 M base = 0.435 (6.55/26.55) = 0.107 M HOCl (aq) + OH-1 (aq) H2O (l) + OCl-1 (aq) 0.132 0.107 -0.107 -0.107 0.025 0 []o []eq HOCl (aq) + H2O (l) H3O+ (aq) + OCl-1 (aq) 0.025 0 0.107 -x +x +x 0.025 x 0.107 3.2x10-8 = x(0.107/0.025) 0 +0.107 0.107 x = 7.48x10-9 pH = 8.13 ii) Calculate the [OH-1] in the flask after the NaOH has been added 1x10-14/7.48x10-9 = 1.34x10-6 M OH-1








