Kansas State University
advertisement

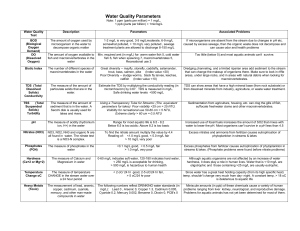
1 Kansas State University Advanced Aquatic Ecology 1 & 2 (BIOL890) Taught through the Division of Biology Instructors: Drs. Walter Dodds and Christopher Guy Fall 1999/Spring 2000 May 24, 2000 _________________________________________________________________________________ Methods Manual for Aquatic Ecological Research: Long-term monitoring of Pottawatomie State Lake II and Kings Creek, Kansas. Authors listed in alphabetical order using last name: Bernot, R., N. Gerlanc, T. Horton, J. Jeffrey, M. Kemp, M. Quist, S. Schrank, D. Stagliano, and C. Zachary. 2 Table of Contents Rationale............................................................................ Study Sites......................................................................... Fall Season Methods (Pott.St.Lake II)........................... Fall Season Methods (Kings Creek)............................... Spring Season Methods (Pott.St.Lake II)....................... Spring Season Methods (Kings Creek)........................... Data Archives and Sample Storage................................ Recommendations for Future Methods.......................... References.......................................................................... Page 3 4 4 10 11 12 12 12 14 Appendix 1 (Species Lists)............................................... Appendix 2 (Zooplankton Images)................................. Appendix 3 (Pott.St.Lake II Equip. List)....................... Appendix 4 (Kings Creek Equip. List ).......................... 16 19 25 29 Figure Captions................................................................. Figure 1 (Pott.St.Lake II)................................................. Figure 2 (Kings Creek)..................................................... 32 33 34 3 Rationale This manual presents methods developed for addressing factors influencing aquatic ecosystem dynamics. Data obtained from methods described herein are important for understanding aquatic ecological processes, and for responsible resource management and conservation. In order to monitor system variability over time, we focus on a variety of abiotic and biotic parameters that can be measured repeatedly at specified sites. This approach provides documentation of short-term system fluctuations, at seasonal scale, and also initiates integrated data collection for long-term system analysis, at decade to century scale. Due to the complexity of physicochemical processes and biotic interactions, we feel it is important to track system characteristics at several levels rather than just a few. A multifaceted research approach will enable investigators to monitor trends over space and time, establish biotic inventories, quantify environmental impacts and ecosystem health, ascertain stream reference sites, and provide baseline data supporting future research and analyses. Our ultimate goal is to conduct research that has the capacity to gain a greater understanding of aquatic ecosystem dynamics, and simultaneously educate future graduate students. Our research design measures one lentic and one lotic system during the spring and fall seasons. Four levels of system monitoring were selected, justification for each follows. Physical & Chemical Parameters. – The abiotic characteristics of water bodies can provide a great deal of insight into the factors affecting the system. Factors such as light, water chemistry, Oxygen (O 2) availability, temperature, and pH can control biotic communities. These factors can also indicate anthropogenic effects on the system. Physical properties of water make it especially vulnerable to pollution inputs. Such inputs can alter the nutrients available and therefore change energy flow within aquatic systems. Water pollution can cause eutrophication, methelhemoglobemia, and toxic algal blooms. Thus, it is essential to maintain an active record of the physical and chemical parameters of water bodies. Plankton. – The plankton community of temperate freshwater lakes varies significantly from year to year as well as from season to season (Lampert and Sommer 1997). Also, vertical structuring of the community at any one time is likely during periods of lake stratification (Leibold and Tessier 1991). Many factors such as resource quantity and quality, food web structure, and physical parameters may ultimately influence these dynamics. Therefore, long-term monitoring of plankton in concert with other biota and abiotic measures, may elucidate dominant mechanisms associated with lentic dynamics. The instantaneous vertical distribution of plankton, before and after summer periods, may also be important for understanding seasonal linkages to other variables. Macroinvertebrates. – Benthic macroinvertebrates dominate the biomass in the sediments of most freshwaters and represent an important component of productivity (Downing 1984). In lentic systems, some of the important relationships among the benthic organisms involve comparisons of the littoral zone with the profundal (Wetzel 1983). The littoral zone typically has a more productive and heterogeneous aquatic community, mainly because of aquatic macrophytes. Aquatic macrophytes support higher invertebrate diversity and abundance when compared to adjacent non-vegetated zones (Dvorak and Best 1982; Iversen et al. 1985). In lotic systems, macroinvertebrates show spatiotemporal variability, are relatively immobile, ubiquitous, highly diverse in species, and have relatively long life cycles. Sampling can be conducted with simple and inexpensive equipment, the taxonomy of many groups is well documented, data analysis methods have been developed and widely used, and pollution responses of many species have been established (Rosenberg and Resh 1993). Therefore, macroinvertebrates have been shown to be effective bioindicators of system impairment (Resh 1995). The macroinvertebrate component of aquatic systems is often considered a contributing force in nutrient cycling, and also a substantial link between primary producers and higher trophic levels. Fish. – As a whole, fishes are important consumers in aquatic food webs. Fishes can be an essential food base for terrestrial mammals, avifauna, and herpetofauna. Physicochemical dimensions and both plankton and macroinvertebrate communities may vary in a way that influence fish communities by changing 4 environmental suitability and food resources. The long-term monitoring approach taken here will aid us in understanding the structure and dynamics of fish communities, and their role in aquatic ecosystem function. Study Sites The lentic site selected for this project was Pottawatomie State Lake II (PSLII), located in Pottawatomie County, Kansas. PSLII is owned by the Kansas Department of Wildlife and Parks (KDWP), and managed primarily for recreation (Figure 1). The lake’s watershed drains tallgrass prairie rangeland and mixed hardwood forest. The lotic site selected for this project was King’s Creek, located on the Konza Prairie Biological Station (KPBS). The permanent study reach lies mid-catchment within a riparian mixed hardwood forest. This stream reach is 100-m in length and is upstream of the public nature trail crossing (Figure 2). KPBS is a 3,487 ha site located in central Geary and Riley counties, Kansas. KPBS is owned by the Nature Conservancy, is managed by Kansas State University-Division of Biology, and is part of the National Science Foundation Long-Term Ecological Research Program. Greater than 90% of the site is tallgrass prairie with limited woodlands along streams. These streams drain large prairie watersheds and exhibit unstable flow regimes and harsh fluctuations in environmental conditions. Fall Season Methods - Pottawatomie State Lake II Aside from fish sample sites, all other samples will be taken from three fixed stations (stations 1, 2, and 3; Figure 1). Station 1 represents the deepest point in the lake, and stations 2 and 3 are located in the two inundated tributaries, “creek arms”. Physical & Chemical Characteristics. – Several parameters will be measured at the established three sites, at one meter intervals within the water column, to determine physicochemical characteristics of the lake. A Hydrolab will be used to measure dissolved oxygen, temperature, redox potential, conductivity, and pH. Other variables measured include light attenuation, ammonium (NH 4+), nitrate (NO3-), phosphorous (PO4-) total nitrogen (TN), and total phosphorous (TP; methods described below). Water samples for determining NH4+, NO3-, PO4-, TN, and TP will be taken using a Van Dorn Bottle. The Van Dorn Sampler has a cord that is marked in 0.5 m increments. Two samples will be taken every meter. 1. 2. 3. 4. 5. 6. 7. 8. 9. Hook suction cups on top. Lower device to appropriate depth. Drop messenger and retrieve Van Dorn Bottle. Pour water sample into Nalgene bottle, overflow bottle with sample water three times. Repeat for sample two. Filter sample one by using hand pump and filtering apparatus (use GF/F filters), and label as filtered, depth, date, initials, and site. Do not filter sample two and label as unfiltered, depth, date, initials, and site. Place samples in cooler. Upon returning to the lab, if samples will be processed in 48 hours, place samples in refrigerator. If processing will occur later, place samples in freezer All chemical analyses, except for the NH4+ analysis, are performed on a Technicon auto-analyzer. The NH4+ analysis will be done by hand using the phenol-hypochlorite method and a Hitachi double-beam spectrophotometer (Solorzano 1969; Greenberg et al. 1998). Light measurements will be made in two ways. First, we will use a Secchi disk to make an indirect measurement of water clarity. 1. Lower Secchi into the water on the shady calm side of the boat until it can barely be detected. 5 2. Record the depth of the disk. The second method is a more direct measurement of light allowing determination of depth at which photoinhibition occurs. A LiCor photometer will be used to measure the available light in mol quanta/m2/sec every meter with depth. It is necessary to take the light readings on a clear day with no clouds. Therefore if day is not clear, do not take measurements with LiCor and rely on Secchi disk information only. 1. 2. Take a light measurement above water in open air. To take a measurement, press “on,” lower the photometer to the required depth, press the “hold” button down, and record measurement. Plankton. – The composite biomass of phytoplankton populations will be estimated by measuring the concentration of chlorophyll a pigments in water samples collected from depths at 1 m intervals. Phytoplankton collection – at 1 m depth intervals 1. 2. 3. 4. Lower Van Dorn to desired depth and send messenger to trap water sample. Filter 100mL of sample water through Whatman GF/F filter (discard unfiltered). Fold filter in half (green on the inside) and wrap in aluminum foil and label. Begin extraction procedure in lab or immediately freeze, and analyze within 2 wks. Extraction – Extraction of chlorophyll a pigments will follow modified procedures of Greenberg et al. (1998) and Sartory and Grobbelaar (1984). Note: extracting and measuring chlorophyll a should be done in subdued lighting (lights off!) to prevent pigment degradation. 1. 2. 3. Place filter in a test tube with 3.5 mL of 90% ethanol. Boil for 5 minutes at 79-80C. Cover all the samples with aluminum foil (to keep them in the dark), and place in the refrigerator for 12-20 hours. Fluorometric Method 1. 2. 3. Centrifuge samples until all the pieces of the filter are on the bottom of the test tube. Use micropipette to carefully pipette liquid into fluorometer tube. Measure sample fluorescence at a sensitivity setting (3x, 10x, or 30x) that will provide a midscale reading. Convert fluorescence readings to concentrations of chlorophyll a (mg/L) by using the appropriate regression equation below: where: y = fluorometer reading x = chlorophyll a concentration (mg/L) 3x: 10x: 30x: 1x: y = 220.1721x – 0.79579 y = 788.6752x – 0.8809 y = 1884.286x – 0.11786 y = 81.07368x + 0.742807 Zooplankton collection – one integrated vertical sample at each site 1. 2. 3. Slowly lower Wisconsin plankton net until it reaches lake bottom. Slowly lift net through water column to the surface. Pour sample from catch container into sampling cup (rinse with 70% EtOH). 6 4. Close sampling cup and label with depth, date, initials, and site. Zooplankton Enumeration – modified from Wetzel and Likens (1991) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Split sample if it is “too dense” (i.e., >500 organisms). To split sample: a. Use ring stand to hold splitter. b. Place a beaker under each splitter tube. c. Pour entire sample into top. d. Wash remaining debris with wash bottle. e. Repeat as needed. Bring sample to 100 mL volume in graduated cylinder. If already >100 mL, settle for 15 minutes, and pipette off excess H20. Pour 90 mL of sample into beaker, then use additional 10 mL to wash remaining debris into beaker (total 100 mL). Stir sample in beaker with volumetric pipette (with 5 mL attachment) for 15 seconds. Take subsample (5 mL) with pipette. Pour subsample into counting wheel groove using glass funnel held by ring stand. Wash remaining debris from pipette or funnel into groove. Let sample settle into groove for a minute or two (note: large amounts of algae may require more settling time). Use dissecting microscope at 12x to count and identify all organisms in groove. Rotate disk slowly and record each organism using a counter. Report zooplankton as number per liter: # of zooplankton / L = C x V’ V” x V”’ where: C = # of organisms counted V’ = volume of the concentrated sample (L) V” = volume counted (L) V”’ = volume of water through which net was towed (L) = r2d ® = radius of net opening, d = depth) Macroinvertebrates. – One benthic sample will be collected at each station. At stations 2 and 3, one quantitative macrophyte sweep will be taken. Collection of these samples will follow physicochemical measurements and plankton collections. Note: Prior to sampling, mix a gallon of 10 % buffered formalin. Pour 185 ml of 37 % Formaldehyde into a 1000 ml graduated-cylinder and fill with water. Repeat four times. Add 5 g CaCO 3 / liter of 10 % formalin = 20 g. Add 1/4 teaspoon of Phloxine B and shake. Benthic Sample: Quantitative estimates of macroinvertebrate density and biomass will be assessed with an Ekman grab sampler. 1. 2. 3. 4. 5. Lower Ekman to the bottom, keep line taut (note depth on the cord). Send messenger down to spring jaws. As the Ekman is retrieved, a bucket will be placed under it at the water surface to avoid losing contents (if jaws did not shut fully, retake sample) Release sample into the bucket, and rinse the inside of the Ekman. Pour onto a 250 m standard sieve; this may take several washes if clogging occurs. A steady flow of water will usually unclog mesh, but tapping the bottom of the sieve often helps. 7 Work the sample to one side of the sieve and wash into an insect pollination bag (poly-bag). Add label and preserve with 10 % buffered formalin stained with Phloxine B. Squeeze bag to remove air, twist and tie an overhand knot. Place in another poly-bag. Labels should include site number, date, Ekman number, depth and initials. Macrophyte Sample: Timed macrophyte sweep samples will be taken with a dipnet to assess the relative abundance and diversity of epiphytic macroinvertebrates in the littoral zone. One sweep will be made for each station, halfway within the macrophyte bed that exists between station and shore (i.e., looking from station perpendicular to shore). Station 1 will not be sampled for macrophytic invertebrates because of its location near the dam which generally lacks macrophytes. 1. 2. 3. A standard (46.5 x 24.5 cm) rectangular dipnet (500 m mesh size) will be forced through the vegetation in a sweeping motion for a period of 30 seconds. Place all macrophytes hanging off the sides into the net and swish water around the net bag to consolidate sample into a corner of the net. Place net contents (dislodged organisms and epiphytes) into a poly-bag, and examine net mesh for remaining organisms. Label and preserve with formalin. Benthic Organism Separation, Enumeration, and Identification: 1. 2. 3. 4. Open poly-bag, fill with water and remove label. Pour sample into bucket and further dilute with water Pour contents onto nested (1 mm and 250 m) sieves, wash sample contents until clean (no more silt going down drain). Separate sieves and rinse contents of 1 mm into a white-bottom enamel pan. Bring pan to an illuminator, remove all organisms with forceps and place them into a scintillation vial filled with 70 % EtOH. Double-check pan for organisms under the dissecting scope. Label vial with site number, date, Ekman replicate, size fraction (i.e., > 1 mm or < 1 mm), subsample fraction (e.g., 1/4, 3/4) and depth. Examine detritus on 250 m sieve, and if loaded with insects (i.e., >200) or too much volume (judgement call, but usually if more than a tablespoon) then split sample with the Zooplankton splitter (see Zooplankton methods). 1. 2. 3. 4. Place subsample in a petri dish and pick all organisms (including zooplankton) under the microscope (10-20x) into scintillation vial w/ 70% EtOH. Identify organisms to the lowest taxon possible and count. For the subsample, multiply the number of each individual taxon by the subsample denominator (e.g., 50 chironomids in a 1/4 sample = 200 for the <1 mm fraction) Combine the <1 mm subsample total with the >1 mm total per taxa and multiply by 43.3 (Ekman area/m2) to get #/m2. To continue with the chironomid example: # of chironomids/m2 =(50 in >1mm + 200 in <1mm) x 43.3= 10,825 Macrophytic Invertebrate Separation, Enumeration, and Identification: 1. 2. 3. 4. 5. 6. Open poly-bag, fill with water and remove label. Pour sample into bucket and further dilute with water, agitate macrophytes. Pour contents onto 500 m sieve (retaining large macrophytes until final rinse), wash sample contents until clean (no more silt going down drain), may take several rinses. Spread macrophytes out in a large tray and examine for invertebrates. Rinse contents of 500 m sieve into a white-bottom enamel pan and pick in proportion of abundance (pick all obvious or large/rare taxa and then the numerous taxa) with a goal of about 200 organisms. Place in a scintillation vial with 70% EtOH and label with site number, method and number, date and position sample was taken from. 8 Fish. – Fish samples will be collected using methods and fixed sampling locations defined by KDWP protocols (Mosher and Willis 1997). Gill nets and trap nets will be set from sunset to sunrise and should not be deployed longer than 24 hours. Nets will be set perpendicular to shore. The gill net complement (i.e., 2.54 cm, 3.81 cm, 6.35 cm) and each trap net represent one unit of effort. A boat-mounted Coffelt electrofishing system with pulsed direct current (DC) will also be used for fish collection. Field size and strength will be standardized for each sampling period. A total of one hour of actual electrofishing time will be conducted during each sampling period (i.e., spring and fall), beginning 0.5 hours after sunset. After each 15 minutes of electrofishing, fish will be brought to shore and processed. All fish will be processed as soon as possible after collection. Each fish will be identified to species and measured to the nearest mm (total length). Weights will be recorded to the nearest gram from 10 fish per centimeter length group per species. Ten or more scales will be removed from target species at the tip of the pectoral fin and below the lateral line (DeVries and Frie 1996). Scale samples will be taken from 10 stock-length fish per centimeter length group for each target species; largemouth bass Micropterus salmoides, bluegill Lepomis macrochirus, redear sunfish Lepomis microlophus, warmouth Lepomis gulosis, white crappie Pomoxis annularis, and black crappie Pomoxis nigromaculatus. Age and growth. – Scale samples will be stored in the laboratory and allowed to air dry. Once dry, scales will be pressed onto acetate slides as follows: 1. 2. 3. 4. 5. Remove five to ten scales from the scale envelope and place them on a new acetate slide (impression slide). The same orientation should be used for all scales (i.e., anterior margin facing in the same direction) and the basal portion of the scale should face up (i.e., shiny side up). Obtain an acetate cover slide (can be reused several times) and place on top of the impression slide. It is often advantageous to wipe the cover slide with your fingers to remove static electricity prior to placing on the impression slide. Place the slides in between the two rollers on the scale press and turn the handle. Check the impression slide to make sure impressions were successful. If successful, place the scales back into the envelope. Label the impression slide with the corresponding species and identification number, and return scales and impression slide into the scale envelope. Note: If scales do not press, it may be necessary to adjust the roller height. Consult Dr. Christopher Guy prior to adjusting roller height. Once all scales have been pressed onto acetate slides, age should be determined using the following guidelines: 1. 2. 3. 4. 5. 6. The same microfiche projector and magnification should be used for all scales. Place the impression slide (impressed side up) into the microfiche projector so that the projected image is oriented with the anterior field facing up. Age should be determined by observing several scales using the following guidelines to determine the presence of an annulus. a. Crowding of circuli. b. Crossing over of circuli in the lateral margins of the scale. c. Consult DeVries and Frie (1996) for further information. Obtain a strip of paper (approximately 279 mm x 51 mm) to mark annuli. Mark the focus (label “F”), each annulus, and the edge (label “E”). All marks should correspond to the anterior portion of the scale and be marked down the middle of the scale. On the piece of paper that you have just marked, write the fish identification number, species, length, and your initials. Place the paper into the scale envelope with the scales and impression slide. 9 Note: It is often helpful to have several people look at the same scale prior to marking scale information (especially if you are inexperienced with aging scales). It is also helpful to proceed from the smallest fish to the largest fish. Once all fish have been aged, the scale information must be put into digital format so that growth can be calculated. This is conducted using DISBCAL located in the Leasure Hall Aquatic Lab. Digitizing of scales should be conducted in the following manner. 1. 2. 3. 4. Turn the digitizing pad on first, then the computer, and then the monitor. Press FN + F10 to enable the monitor. You will then enter the following commands. a. (1)–Measure bony parts <ENTER> b. Enter the filename without an extension (must be less than 8 characters) <ENTER>. c. (2)–Fishnumber, Length, Age <ENTER>; if you are adding fish to an existing file you must enter (2)–Append <ENTER>. d. (3)–Digitizer only <ENTER>. e. Digitize the upper left corner of the paper the contains the template and then the lower right corner (use the cross hairs on the mouse). The program will ask you to repeat this process once. If you are consistent, the program will continue, otherwise you will have to redo this process. f. Enter the magnification; everything is with the numeric pad on the digitizer not on the keyboard. Now you are ready to digitize the scale information removing the paper from the envelope and placing it anywhere on the digitizing pad. a. Enter the fish identification number (i.e., using the digitizing pad) <RETURN>. b. Enter the length <RETURN>. c. Enter the age <RETURN>. d. Put the cross hairs over the focus and press one of the buttons. Continue with the annular marks and the edge. If you entered the age correctly, the program will automatically provide the correct number of annuli that you must enter. e. If everything is correct (i.e., fish number, length, age) then press <RETURN>, otherwise press <ESC> to redo. f. When you are ready to exit, enter the measurements for the last fish and press <Y>–exit with add. g. To exit the program enter 5–terminate program <ENTER> to exit back to the DOS prompt. Note–The first time you enter DISBCAL the program will ask you for the magnification, but will skip this step when reopening a file. If you are adding to an existing file, be sure to enter the correct drive and file name (i.e., the floppy drive instead of the hard drive). The scale measurement information is now ready to be analyzed. 10 Fall Season Methods - Kings Creek Water samples are taken three times a week by the LTER program at the public nature trail crossing. Any information regarding the chemical concentrations of the stream can be obtained by contacting Walter Dodds (Bushnell Hall). Macrohabitat. – Each macrohabitat (i.e., pool, riffle, run) will have three transects set perpendicular to stream-flow. Transects will be placed at 0.25, 0.5, and 0.75 of macrohabitat length. Three widths (nearest decimeter; one per transect) and one length (m) for each macrohabitat will be measured with a rangefinder (Impulse 2). Any logs, boulders, or debris should be included in the width measurement; however, accumulations of inorganic sediments greater than 0.31 m in width will be considered islands and should not be included in the stream width measurement (Platts et al. 1983). Current velocity (m/s), depth (m), and substrate type, will be measured at 20, 40, 50, 60, and 80% of the stream width at each transect (Platts et al. 1983). Current velocity will be measured using a Marsh-McBirney Flo-Mate Model 2000 flowmeter. At depths less than 0.75 m, one measurement of the velocity will be taken at 60% of the water depth (Buchanan and Somers 1969; McMahon et al. 1996). At depths greater than 0.75 m, velocity will be measured at 20% and 80% of the water depth (Buchanan and Somers 1969). Stream depth will be measured using a calibrated top-set wading rod to the nearest centimeter. Substrate will be measured using the point-transect method and will be classified using a modified Wentworth system (McMahon et al. 1996), except for the inclusion of a bedrock category and the pooling of sand categories. In areas where visual examination of substrate is hindered by turbidity or depth, substrate particle size will be estimated by touch with the wading rod (Platts et al. 1983). Substrate categories are as follows: bedrock or boulder (> 256 mm), cobble (65 - 256 mm), pebble (32 - 64 mm), gravel (2 - 32 mm), sand (0.0625 - 2 mm), silt (0.0039 - 0.0625 mm), clay (< 0.0039 mm). Mesohabitat. – The area of mesohabitats (m2) will be measured for each macrohabitat. Mesohabitat categories include: log, log complex, aquatic vegetation, brush pile, boulder, overhanging vegetation, and root wad. To be classified as one of the previously mentioned categories, the habitat in question must have a minimum width and length of 0.1 m. Two widths and two lengths from each mesohabitat will be recorded. To remain consistent, classification of mesohabitats will be conducted by the student responsible for the ongoing, long-term fish monitoring study (contact Dr. Christopher Guy). Canopy Cover. – Canopy cover of the stream reach will be determined using a densiometer. At the center of each macrohabitat, four measurements will be taken: facing downstream, facing upstream, and facing each bank. The densiometer is held chest-level and the numbers of squares with light (no vegetation) are counted. Percent canopy cover is then calculated from the following equation: % cover = average number of squares with light x 96 x 1.04 These directions are also on the back of the densiometer and can be referred to in the field. Periphyton. – Periphyton biomass will be determined by obtaining the ash-free dry mass (AFDM) and chlorophyll a concentrations of collected samples. This involves drying the collected samples to a constant weight, oxidizing them in a muffle furnace and re-weighing the oxidized samples. The loss in weight upon oxidation is referred to as the AFDM. Prior to field sampling, filters should be ashed in the muffle furnace (550°C, 3 hours) and their weight recorded. These pre-weighed filters will be used to filter the biomass samples. Periphyton will be collected by selecting four relatively flat rocks from each of two riffles within the reach, or 8 rocks encompassing total available riffle habitat if no independent riffles exist. Collection is performed by placing an epilithon sampler on a rock to form a sealed section of the rock. The sealed section is then agitated by brushing to displace all periphyton and then siphoned off using a turkey baster and placed into the sampling container. The rock should be washed and the liquid within the sealed section collected several times to ensure all of the periphyton is obtained. Furthermore, it is important to rinse the brush in the sampling container to collect all of the periphyton left on the bristles. Two of the samples from each riffle site will be used for biomass calculations. These samples will be filtered onto pre-weighed Whatman GF/C filters and placed in the drying oven. The samples are 11 weighed, after thoroughly drying, and placed into the muffle furnace (550°C, 3 hours). Once the samples are ashed, they are re-wet by gently squirting with water and again allowed to dry in the drying oven. Finally, when dry, they are again weighed. AFDM is obtained using the following calculation: AFDM = (dry wt - filter wt) - (ash wt - filter wt) The average AFDM is then scaled up to the whole stream using the area of the epilithon sampler. The remaining two samples from each of the riffles will be used to determine the chlorophyll a concentrations within the reach. The samples will again be filtered onto a Whatman GF/C filter (no preweighing necessary) and then folded and wrapped in foil. These samples will be placed in the freezer upon return from the field for future chlorophyll a extractions (see PSFLII methods for extraction procedures). Macroinvertebrates. – Riffle macroinvertebrates will be quantitatively sampled by taking 3 replicate Surber samples. Placement of the Surbers should encompass the total available riffle habitat. Multi-habitat qualitative collections will be made with a standard-sized dipnet (one composite of 20 jabs in proportion to riffle, pool, and run availability). Surber samplers should be placed in water at least 12 cm deep and all substrata should be dislodged and rubbed clean; keep large substrate out of the surber’s bag. Dipnet jabs in pools should focus on submerged woody debris, roots, undercut banks, and macrophytes. Dipnet jabs in riffles should focus on cobble and pebble substrate. All dipnet jabs should be approximately 0.5 m in length. All samples will be placed in labeled poly bags, immediately preserved with Phloxine-B stained 10% formalin, and later sorted and identified to the lowest taxon possible. These methods follow the updated version of Plafkin et al. (1989). Plastic paper labels should include date, site, and method (dipnet, surber 1, 2, or 3). In the laboratory, dipnet samples will be sorted using a two-phase processing method and surber samples will be sorted using a nested-sieve method. For both methods, a 200 organism fixed-count technique is typically employed, where processing continues until the target number is achieved. Although a 100 organism count is generally sensitive to species richness and abundance, the power of discriminating differences among assemblages can be reduced (Barbour and Gerritsen 1996; Courtemanch 1996; Vinson and Hawkins 1996). Therefore, attempts will be made to obtain 200 organisms for all samples. A dipnet sample is first washed onto a 500 m sieve and then rinsed into a sorting tray. The twophase processing method first searches the entire sample for large and rare organisms, then a subsample based upon a fraction of the whole is searched. If organisms are few in the first 1/4 of the second phase, a second 1/4 should be processed, and so on. A surber sample should be washed onto nested 1mm and 250 m sieves. The entire collection from the 1 mm sieve is first picked, then subsampling of the 250 m sieve follows until obtaining at least 200 organisms (see PSFLII macroinvertebrate section for technique details). Fish. – The Coffelt backpack unit should be set on DC current, at 30 cycles per second (cps), between 100300 volts and 3-5 amps (depending on the conductivity of the water, volts may be adjusted accordingly to obtain 3-5 amps). The generator output will be set on 300 volts (denoted as 300 VA on backpack unit). Each macrohabitat will be sampled independently. Electrofishing will begin at the downstream end of each macrohabitat and proceed upstream, ending when the uppermost portion of the macrohabitat is reached. The anode will be moved in a smooth, arc-like motion from bank to bank. Two assistants will net fish on either side of the backpack unit and place them in a bucket that is approximately half-full of water. Total time electrofishing will be recorded for each macrohabitat (read and reset timer on the backpack unit–with the switch on the anode–prior to electrofishing each macrohabitat). Fish from each macrohabitat will be processed separately. All fish will be sorted by species and processed as soon as possible. Total length (mm) will be measured from the first 100 fish of each species, subsequent fish of that species will be counted. All fish will be kept separate based on macrohabitat type and number. Species name abbreviations and data sheets should follow standards in Appendix. All fish will be returned to the macrohabitat from which they were collected. Spring Season Methods - Pottawatomie State Lake II Physical & Chemical Characteristics. – Only site one will be assessed. Methods same as fall season. 12 Plankton. – Only site one will be assessed. Methods same as fall season. Macroinvertebrates. – Only site one will be assessed. Methods same as fall season, except no macrophyte samples are taken. Fish. – Fall season methods will not be performed, the following exercise will be used instead. Population Estimate of Largemouth Bass. – The Peterson Index will be used to estimate the population size of largemouth bass in PSFLII. Two sampling periods will be required for this mark-recapture technique (i.e., two nights with a two or three day interval). Night-time boat electrofishing will be used for collection of all fish. Night-one: Largemouth bass will be collected using four, 15 minute electrofishing runs. Methods for boat electrofishing can be found in the previous PSFLII section. After each run, largemouth bass will be marked by removing a slanted clip from the lower portion of the caudal fin (Guy et al. 1996). Fish will be returned as soon as possible to the midpoint of the electrofishing run. The number of largemouth bass marked will be counted and recorded. Night-two: Largemouth bass will be collected and examined for marks. Four, 15 minute runs will be conducted in the same locations used during the marking effort. Prior to examining largemouth bass for marks, all individuals should review Guy et al. (1996). Largemouth bass collected during each run will be held in the live-well until all four runs are completed. Then fish will be examined for marks. The number of fish with marks (recaptures) will be counted, as well as the total number of bass collected. Populations estimate. The formula for estimating the population of largemouth bass is: N = MC/R N = estimate of population size (N) M = # of fish marked C = total # of fish collected on night-two R = # of recaptures This estimate applies to the population present during the first sample period. It is based on the assumption that the proportion of marked fish in the second sample estimates the proportion of fish in the total population. This index can give biased estimates if sample size is low, however modification of the equation can decrease this bias (see Bailey’s modification; Van Den Ayle 1993). Spring Season Methods - Kings Creek Same as fall season methods. Data Archives and Sample Storage Notebooks and data sheets for all Advanced Aquatic Ecology classes will be archived in a filing cabinet in room 212, Leasure Hall. Electronic versions of this manual, and all raw data and metadata are available on the internet at http:/www-personal.ksu.edu/~wkdodds/aquatecol/ advaquat.html. Fish scales collected during sampling will be stored in room 212, Leasure Hall. All water and zooplankton samples will be disposed of after processing. At this time, aquatic macroinvertebrates are being donated for teaching purposes to the Department of Entomology, Waters Hall. Recommendations for Future Methods Due to logistical constraints there are several techniques that we were unable to incorporate into this project, yet we believe that some may be warranted. For the lentic site, bathymetric mapping would better portray water volume, depth, and other macrohabitat features. Also for the lentic site, consideration should be given to future faunal surveys for tributary fish, lake amphibians and reptiles, adult Odonates, and water birds. For the lotic site, the stream channel should be characterized in terms of other physical features, e.g., in a way that would show channel movement over time (see Harrelson et al. 1994). Also for the lotic site, aspects of crayfish and amphibian influences on stream system function should be addressed. 13 Some of these suggestions might be possible if viewed as multi-annual endeavors rather than mandatory semi-annual surveys; flexible to new students’ specialties. Implicit to this project endeavor are large data files covering both biotic and abiotic variables for two sites. Providing a way to have these data widely and easily accessible to users is an objective that will require future refinement. Having protocols for data archival and backup should be established. Data files for 1999 exist as independent constructions, with designs that are varied. Although these files can be brought into a relational database (a database of databases), the overall design will lack some interconnectivity necessary for comprehensive queries. 14 References Barbour, M. T., and J. Gerritsen. 1996. Subsampling of benthic samples: a defense of the fixed-count method. Journal of the North American Benthological Society 15(3):386-391. Buchanan, T. J., and W. D. Somers. 1969. Discharge measurements at gaging stations. United States Geological Survey, Techniques of Water-Resources Investigations, Book 3, Washington D.C. Courtemanch, D. L. 1996. Commentary on the subsampling procedures used for rapid bioassessments. Journal of the North American Benthological Society 15(3):381-385. Cross, F. B., and J. T. Collins. 1995. Fishes in Kansas, 2 nd edition. University of Kansas, Lawrence. DeVries, D. R., and R. V. Frie. 1996. Determination of age and growth. Pages 483-512 in B. R. Murphy and D. W. Willis, editors. 1996. Fisheries techniques, 2 nd edition. American Fisheries Society, Bethesda, Maryland. Downing, J. A. 1984. Sampling the benthos of standing waters. Pages 87-130 in J. A. Downing and F. H. Rigler, editors. A Manual on Methods for the Assessment of Secondary Productivity, 2 nd edition. Blackwell, Oxford. Dvorak, J., and E. P. Best, 1982. Macro-invertebrate communities associated with the macrophytes of Lake Vechten: structural and functional relationships. Hydrobiologia 95:115-126. Greenberg, A. E., L. S. Clesceri, and A. D. Eaton. 1998. Standard Methods for the Examination of Water and Wastewater, 20th edition. American Public Health Association joint publication with American Water Works Association and Water Environment Federation, New York City. Guy, C. S., H. L. Blankenship, and L. A. Nielsen. 1996. Tagging and marking. Pages 353-383 in B. R. Murphy and D. W. Willis, editors. 1996. Fisheries techniques, 2nd edition. American Fisheries Society, Bethesda, Maryland. Harrelson, C. C., C. L. Rawlins, and J. P. Potyondy. 1994. Stream channel reference sites: an illustrated guide to field technique. USDA, Gen. Tech. Rep. RM-245. Fort Collins, CO. Iversen, T. M., J. Thorp, T. Hansen, J. Lodel and J. Olsen, 1985. Quantitative estimates and community structure of invertebrates in a macrophyte rich stream. Archives Hydrobiologia 102:291-301. Lampert, W., and U. Sommer. 1997. Limnoecology: the ecology of lakes and streams. Oxford University Press, New York City. Leibold, M. A., and A. J. Tessier. 1991. Contrasting patterns of body size for Daphnia species that segregate by habitat. Oecologia 86:342-346. Merritt, R. W., and K. W. Cummings. 1996. An introduction to the aquatic insects of North America, third edition. Kendall Hunt Publishing Company, Dubuque, Iowa. McMahon, T. E., A.V. Zale, and D. J. Orth. 1996. Aquatic habitat measurements. Pages 83-120 in B. R. Murphy and D. W. Willis, editors. Fisheries techniques, 2nd edition. American Fisheries Society, Bethesda, Maryland. Mosher, T., and D. W. Willis. 1997. Fish survey techniques for small lakes and reservoirs, third edition. Kansas Department of Wildlife and Parks, Pratt, Kansas. 15 Pennak, R. W. 1988. Fresh-water invertebrates of the Unites States: protozoa to mollusca. Wiley, New York. Pfleiger, W.L. 1997. The fishes of Missouri, revised edition. Missouri Department of Jefferson City. Conservation, Plafkin, J. L., M. T. Barbour, K. D. Porter, S. K. Gross, and R. M. Hughes. 1989. Rapid bioassessment protocols for use in streams and rivers. EPA/444/4-89/001, USEPA, Office of Water Regulation and Standards, Washington, D.C. Platts, W. S., and W. F. Megahan, and G. W. Minshall. 1983. Methods for evaluating stream, riparian, and biotic conditions. U. S. Forest Service General Technical Report INT-138. Resh, V. H. 1995. Freshwater benthic macroinvertebrates and rapid assessment procedures for water quality monitoring in developing and newly industrialized countries. Pages 167-177 in W. S., Davis, and T. P. Simon, editors. Biological assessment and criteria: tools for water resource planning and decision making. Lewis Publishers, Boca Raton, Florida. Rosenberg, D. M., and V. H. Resh. 1993. Introduction to freshwater biomonitoring and benthic macroinvertebrates. Pages 1-8 in D. M. Rosenberg, and V. H. Resh, editors. Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York. Sartory, D. P., and J. U. Grobbelaar. 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:117-187. Solorzano, L. 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology & Oceanography 14:799-801.Van Den Ayle, M. J. 1993. Dynamics of exploited fish populations. Pages 105-134 in C. C. Kohler and W. A. Hubert, editors. Inland fisheries management in North America. American Fisheries Society, Bethesda, Maryland. Vinson, M. R., and C. P. Hawkins. 1996. Effects of sampling area and subsampling procedure on comparisons of taxa richness among streams. Journal of the North American Benthological Society 15(3): 392-399. Wetzel, R. G., 1983. Limnology, 2nd edition. Saunders College Publishing, Philadelphia. Wetzel, R. G., and G. E. Likens. 1991. Collection, enumeration, and biomass of zooplankton. Pages 167178 in Limnological Analyses 2nd edition. Springer-Verlag, New York. 16 Appendix 1: Fish species Lists 17 Pottawatomie State Lake II fish species list Species Scientific name Abbreviation Dorosoma cepedianum GIZZ Notemigonus crysoleucas GOSH Channel catfish Ictalurus punctatus CCF Flathead catfish Pylodictus olivaris FHCF Green sunfish Lepomis cyanellus GRSF Warmouth Lepomis gulosus WAR Bluegill Lepomis macrochirus BLGL Redear sunfish Lepomis microlophus RESF Herrings (Clupeidae) Gizzard shad Minnows (Cyprinidae) Golden shiner Catfishes (Ictaluridae) Sunfishes (Centrarchidae) Hybrid sunfish HYB Smallmouth bass Micropterus dolomieu SMB Largemouth bass Micropterus salmoides LMB White crappie Pomoxis annularis WHITE Black crappie Pomoxis nigromaculatus BLACK Walleye Stizostedion vitreum WAE Saugeye Stizostedion canadense x Stizostedion vitreum SEYE 18 Kings creek fish species list Species Minnows (Cyprinidae) Central stoneroller Red shiner Common shiner Redfin shiner Sand shiner Rosyface shiner Southern redbelly dace Bluntnose minnow Fathead minnow Creek chub Suckers (Catostomidae) White sucker Shorthead redhorse Catfishes (Ictaluridae) Scientific name Abbreviation Campostoma annomalum Cyprinella lutrensis Luxilus cornutus Lythrurus umbratilis Notropis ludibundus Notropis rubellas Phoxinus erythrogaster Pimephales notatus Pimephales promelas Semotilus atromaculatus CEST RESH COSH RFSH SASH ROSH SRDA BLMI FAMI CRCH Catostomus commersonii Moxostoma macrolepidotum WHSU SHRE Black bullhead Yellow bullhead Slender madtom Stonecat Livebearers (Poeciliidae) Mosquitofish Sunfishes (Centrarchidae) Ameiurus melas Ameiurus natalis Noturus exilis Noturus flavus BLBU YEBU SLMA STON Gambusia affinis MOSQ Green sunfish Orangespotted sunfish Bluegill Longear sunfish Largemouth bass Perches (Percidae) Johnny darter Orangethroat darter Lepomis cyanellus Lepomis humilis Lepomis macrochirus Lepomis megolotis Micropterus salmoides GRSU ORSU BLUE LOSU LABA Etheostoma nigrum Etheostoma spectabile JODA ORDA 19 Appendix 2: Zooplankton Images 20 21 22 23 24 25 Appendix 3: Pottawatomie State Lake II Equipment List 26 PSFL2 Equipment Checklist General notebook sharpies pencils data sheets clipboards Physical & Chemical Characteristics Field (Dodds Lab) 40 acid washed water sample bottles GF/C filters (27mm) filtering apparatus hydrolab w/ laptop cooler w/ ice light meter Van Dorn Bottle Secchi Disk Lab (Dodds Lab) 80 acid washed test tubes chemicals for ammonium assay (see Standard Methods for Analysis of Water and Wastewater) spectrophotometer pipettes autoanalyzer Phytoplankton Field (Dodds Lab) Van Dorn Sampler hand vacuum pump 47mm Whatman GF/F filters aluminum foil Lab (Dodds Lab) test tubes 90% ethanol centrifuge Fluorometer (w/ high intensity F4T.5 lamp) photomultiplier tube R-446 (red sensitive) micropipette small light source Zooplankton Field (Dodds Lab) Wisconsin plankton net -- 30 cm diameter, 80m mesh rope marked in 0.5m intervals (from ring of net) sampling cups with lids squeeze bottle w/ 70% ethanol Lab (Dodds Lab) ring stand w/ clamp dissecting probes taxonomic keys 100mL graduated cylinder squeeze bottle w/ water binocular dissecting microscope plankton counting wheel zooplankton splitter volumetric pipette w/ 5mL attachment 2 beakers > 100mL glass funnel 27 Invertebrates Field (Dodds Lab unless otherwise indicated) Ekman grab sampler (15.2 x 15.2cm) 3-5 gallon buckets Sieves: 250, 500 m (500 sieve from entomology or Guy Lab) 2 Wash bottles 36 poly-bag (entomology department or Guy Lab) 10% Buffered Formalin w/ Phloxine B Labels Standard Rectangular dipnet (500m mesh) Lab (Dodds Lab) Binocular Dissecting Microscope Illuminator Sieves (1mm, 500 & 250m) Wash Bottles Zooplankton Splitter 70% EtOH White-bottom enamel pans Forceps 500 ml beakers Scintillation vials Taxonomic materials (Merritt & Cummings 1996; Pennak 1988) Petri Dishes Fish Boat (Guy Lab) live well, 3 five gallon buckets type I personal flotation device for each boat occupant 1 type IV throw able flotation device safety kit (includes: fire extinguisher, whistle, extra boat plug, flashlight, extra batteries, first aid kit, visual distress signal, 30 feet of extra rope) gas, oil, boat keys, anchor, oar Electrofishing (Guy Lab) Electrofishing Boat generator Coffelt variable voltage pulsator boom sphere dip nets: 2 long handle, 1 short handle 3 pair of rubber gloves waders for each boat occupant Boat Lights (Coop Shed) 2 Lanterns (for fish work up; Guy’s Lab) 3 Head Lamps (personal item) Gill Netting (KDWP unless indicated) monofilament gill net complement: 1 net each of 1", 1.5", and 2.5" mesh; net dimensions 100' long and 8' deep. For additional information on gill net specifications see Mosher and Willis (1997). 2 gill net picks (Guy Lab) 6 weights 6 floats 6-15 meter long ropes Trap Nets (KDWP) 4 trap nets: 4' high and 5' wide with 0.5" bar mesh. Additional information of trap net specifications can be found in Mosher and Willis (1997). 28 4 floats 4 anchors 4 stakes Work-up (Guy Lab) digital scale: 0 to 1200 grams capacity analog scale: 0 to 25 kg capacity measuring board: max length 1m knife stamped coin envelopes weighing pan Wind Blocking Device Extra Batteries for scales 29 Appendix 4: Kings Creek Equipment List 30 KINGS CREEK Equipment Checklist General notebook pencils sharpies datasheets clipboard Habitat Field (Guy Lab) Measuring tape (100-m) Calibrated top-set wading rod Marsh-McBirney Flo-Mate, Model 2000 Flowmeter Rangefinder (Impulse 2) Spherical Densiometer Periphyton Field (Dodds Lab unless otherwise indicated) plastic sample bags large GF/C filters hand filtering apparatus (2) foil weigh dishes foil squares epilithon sampler stiff brush densiometer (Guy lab) pee cups (or equivalent sampling container) Lab (Dodds Lab) drying over electronic scale Macro-invertebrates Field (Dodds Lab) Surber Sampler (30.5cm x 30.5 cm; 250 Fm mesh) Rectangular Dipnet (500 Fm mesh) 5 gallon bucket (2) Wash bottles (2) Phloxine B-stained 10% formalin Poly-bags (16) Labels Neoprene gloves (optional) Lab (Dodds Lab) Binocular Dissecting Microscope Illuminator Sieves (1mm, 500 & 250m) Wash Bottles Zooplankton Splitter 70% EtOH White-bottom enamel pans Forceps 500 ml beakers Scintillation vials Taxonomic materials (Merritt & Cummings 1996; Pennak 1988) Petri Dishes 31 Fish Field (Guy Lab) Coffelt Mark-10 backpack electrofisher with circular anode (yellow handle) 2.5-gallon gasoline container; mixed 50:1 with 2-cycle Honda oil 3 dip nets (3/16" mesh; fiberglass handle) 4 pairs of lineman’s rubber gloves 4 five-gallon buckets 2 small green aquaria nets Measuring board Copy of Cross and Collins (1995) and Pfleiger (1997) 32 Figure Caption Figure 1.–Location of study sites (1-3) in Pottawatomie State Lake II. Black rectangles denote boat ramp, dashed lines denote gravel roads, dotted lines denote tributaries of the lake, gray rectangles denote rip-rap fishing piers, black circles denote site locations, and red circle denotes the dam outlet. Figure 2.–Location of study reach on Kings Creek. Black rectangle denotes stream bridge crossing, black triangle denotes the most downstream end of the study reach, dotted line denotes the public nature trail, and dashed line denotes Kings Creek. 33 34