Exploring Chemical Reactions & Fractals Assessment Key
advertisement

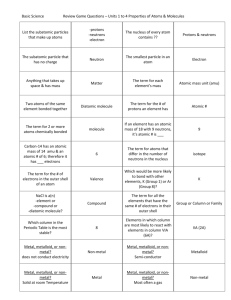
Exploring Chemical Reactions & Fractals Assessment Key 1. Identify any clues in the experiment described below that indicate a chemical reaction may have occurred. Scientists took 15mL of one liquid and mixed it with 35mL of a different liquid. A yellow solid began forming in the liquid mixture. The mixture cooled down from 20ºC to 4ºC during the first 10 minutes after being mixed. A new substance in the form of a yellow solid formed. A solid that forms in solution as the result of a chemical reaction is called a precipitate. The temperature of the solution decreased indicating heat energy had to be absorbed for the reaction to proceed. Heat was a reactant and the reaction should be classified as endothermic. 2. What kinds of atoms can form an ionic bond? a) Two metal atoms b) Two nonmetal atoms c) One metal and one nonmetal atom d) One nonmetal and one semi-metal atom 3. What kinds of atoms can form a covalent bond? a) Two metal atoms b) Two nonmetal atoms c) One metal and one nonmetal atom d) One nonmetal and one semi-metal 4. A simple equation often used to represent the formation of rust is 4Fe + 3 O2 2Fe2O3 a) Is iron a metal or nonmetal? metal b) Is oxygen a metal or nonmetal? nonmetal c) What kind of bond exists between iron and oxygen in the compound that forms? A covalent bond. Exploring Chemical Reactions & Fractals Assessment Key http://MathInScience.info ©MathScience Innovation Center 2007 5. Use the periodic table to decide which of the following compounds are ionic and which are covalent. Place yes or no in the columns to the right of each compound to indicate which type of bond forms. Formula CaCl2 CO2 Al2O3 MgS NaF AsI3 Covalent Bonding No Yes No No No Yes Ionic Bonding Yes No Yes Yes Yes No 6. Some chemical reactions are exothermic because a) the substances are mixed in a sealed container. b) there is not enough energy stored in the bonds of the reactants to form the product(s). c) there is more energy stored in the bonds of the reactants than is needed to form the product(s). d) the reaction vessel is heated on a hot plate. 7. A friend in another science class overhears you talking about fractals and asks what a ‘fractal’ is and why fractals are important to scientists. Record your response below. Fractals are geometric figures just like rectangles, squares and circles but fractals have irregular shapes and surfaces that cannot be measured and analyzed using traditional methods and tools. Fractals are composed of patterns that are continuously repeated such that as you increase the magnification the smaller structures look like the whole. For example, a head of cauliflower looks like one of the individual florets. Random behavior of atoms and molecules at a microscopic scale sometimes leads to the formation of these complex predictable patterns at the macroscopic scale that we identify as fractals. Studying the patterns formed or measuring the fractal dimension of these structures helps scientists understand and predict natural phenomena such as stream and coastal erosion, fingering instabilities during oil extraction and the diffusion of drugs within the human body. Exploring Chemical Reactions & Fractals Assessment Key http://MathInScience.info ©MathScience Innovation Center 2007